Impact of soluble epoxide hydrolase and epoxyeicosanoids on human health
- PMID: 23020295
- PMCID: PMC3578707
- DOI: 10.1146/annurev-pharmtox-011112-140244
Impact of soluble epoxide hydrolase and epoxyeicosanoids on human health
Abstract
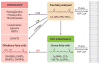
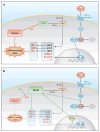
The presence of epoxyeicosatrienoic acids (EETs) in tissues and their metabolism by soluble epoxide hydrolase (sEH) to 1,2-diols were first reported 30 years ago. However, appreciation of their importance in cell biology and physiology has greatly accelerated over the past decade with the discovery of metabolically stable inhibitors of sEH, the commercial availability of EETs, and the development of analytical methods for the quantification of EETs and their diols. Numerous roles of EETs in regulatory biology now are clear, and the value of sEH inhibition in various animal models of disease has been demonstrated. Here, we review these results and discuss how the pharmacological stabilization of EETs and other natural epoxy-fatty acids could lead to possible disease therapies.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

