Epigenetic mechanisms of depression and antidepressant action
- PMID: 23020296
- PMCID: PMC3711377
- DOI: 10.1146/annurev-pharmtox-010611-134540
Epigenetic mechanisms of depression and antidepressant action
Abstract
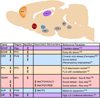
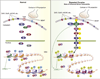
Epigenetic mechanisms, which control chromatin structure and function, mediate changes in gene expression that occur in response to diverse stimuli. Recent research has established that environmental events and behavioral experience induce epigenetic changes at particular gene loci and that these changes help shape neuronal plasticity and function and hence behavior. Some of these changes can be stable and can even persist for a lifetime. Increasing evidence supports the hypothesis that aberrations in chromatin remodeling and subsequent effects on gene expression within limbic brain regions contribute to the pathogenesis of depression and other stress-related disorders such as post-traumatic stress disorder and other anxiety syndromes. Likewise, the gradually developing but persistent therapeutic effects of antidepressant medications may be achieved in part via epigenetic mechanisms. This review discusses recent advances in our understanding of the epigenetic regulation of stress-related disorders and focuses on three distinct aspects of stress-induced epigenetic pathology: the effects of stress and antidepressant treatment during adulthood, the lifelong effects of early-life stress on subsequent stress vulnerability, and the possible transgenerational transmission of stress-induced abnormalities.
Figures




References
-
- de Kloet ER, Joels M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev Neurosci. 2005;6:463–475. - PubMed
-
- Chrousos GP. Stress and disorders of the stress system. Nat Rev Endocrinol. 2009;5:374–381. - PubMed
-
- Von Holst D. The concept of stress and its relevance for animal behavior. Advances in the Study of Behavior. 1998:1–131.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

