Intravenous multipotent adult progenitor cell therapy after traumatic brain injury: modulation of the resident microglia population
- PMID: 23020860
- PMCID: PMC3546881
- DOI: 10.1186/1742-2094-9-228
Intravenous multipotent adult progenitor cell therapy after traumatic brain injury: modulation of the resident microglia population
Abstract
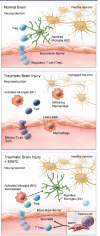
Introduction: We have demonstrated previously that the intravenous delivery of multipotent adult progenitor cells (MAPC) after traumatic brain injury affords neuroprotection via interaction with splenocytes, leading to an increase in systemic anti-inflammatory cytokines. We hypothesize that the observed modulation of the systemic inflammatory milieu is related to T regulatory cells and a subsequent increase in the locoregional neuroprotective M2 macrophage population.
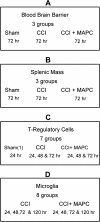
Methods: C57B6 mice were injected with intravenous MAPC 2 and 24 hours after controlled cortical impact injury. Animals were euthanized 24, 48, 72, and 120 hours after injury. In vivo, the proportion of CD4(+)/CD25(+)/FOXP3(+) T-regulatory cells were measured in the splenocyte population and plasma. In addition, the brain CD86(+) M1 and CD206(+) M2 macrophage populations were quantified. A series of in vitro co-cultures were completed to investigate the need for direct MAPC:splenocyte contact as well as the effect of MAPC therapy on M1 and M2 macrophage subtype apoptosis and proliferation.
Results: Significant increases in the splenocyte and plasma T regulatory cell populations were observed with MAPC therapy at 24 and 48 hours, respectively. In addition, MAPC therapy was associated with an increase in the brain M2/M1 macrophage ratio at 24, 48 and 120 hours after cortical injury. In vitro cultures of activated microglia with supernatant derived from MAPC:splenocyte co-cultures also demonstrated an increase in the M2/M1 ratio. The observed changes were secondary to an increase in M1 macrophage apoptosis.
Conclusions: The data show that the intravenous delivery of MAPC after cortical injury results in increases in T regulatory cells in splenocytes and plasma with a concordant increase in the locoregional M2/M1 macrophage ratio. Direct contact between the MAPC and splenocytes is required to modulate activated microglia, adding further evidence to the central role of the spleen in MAPC-mediated neuroprotection.
Figures








References
-
- Olson JK, Miller SD. Microglia initiate central nervous system innate and adaptive immune responses through multiple TLRs. J Immunol. 2004;173:3916–3924. - PubMed
-
- Beck KD, Nguyen HX, Galvan MD, Salazar DL, Woodruff TM, Anderson AJ. Quantitative analysis of cellular inflammation after traumatic spinal cord injury: evidence for a multiphasic inflammatory response in the acute to chronic environment. Brain. 2010;133:433–447. doi: 10.1093/brain/awp322. - DOI - PMC - PubMed
-
- Smith HS. Activated microglia in nociception. Pain Physician. 2010;13:295–304. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

