CMR of microvascular obstruction and hemorrhage in myocardial infarction
- PMID: 23021401
- PMCID: PMC3514126
- DOI: 10.1186/1532-429X-14-68
CMR of microvascular obstruction and hemorrhage in myocardial infarction
Abstract
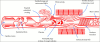
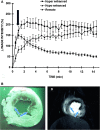
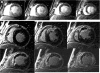
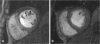
Microvascular obstruction (MO) or no-reflow phenomenon is an established complication of coronary reperfusion therapy for acute myocardial infarction. It is increasingly recognized as a poor prognostic indicator and marker of subsequent adverse LV remodeling. Although MO can be assessed using various imaging modalities including electrocardiography, myocardial contrast echocardiography, nuclear scintigraphy, and coronary angiography, evaluation by cardiovascular magnetic resonance (CMR) is particularly useful in enhancing its detection, diagnosis, and quantification, as well as following its subsequent effects on infarct evolution and healing. MO assessment has become a routine component of the CMR evaluation of acute myocardial infarction and will increasingly play a role in clinical trials of adjunctive reperfusion agents and strategies. This review will summarize the pathophysiology of MO, current CMR approaches to diagnosis, clinical implications, and future directions needed for improving our understanding of this common clinical problem.
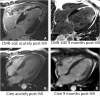
Figures







References
-
- Judd RM, Lugo-Olivieri CH, Arai M, Kondo T, Croisille P, Lima JA, Mohan V, Becker LC, Zerhouni EA. Physiological basis of myocardial contrast enhancement in fast magnetic resonance images of 2-day-old reperfused canine infarcts. Circulation. 1995;92:1902–10. doi: 10.1161/01.CIR.92.7.1902. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

