Paracrine and autocrine interactions in the human islet: more than meets the eye
- PMID: 23022232
- PMCID: PMC3570628
- DOI: 10.1016/j.semcdb.2012.09.007
Paracrine and autocrine interactions in the human islet: more than meets the eye
Abstract
The pancreatic islet secretes the hormones insulin and glucagon to regulate glucose metabolism. To generate an adequate secretory response, islet endocrine cells must receive multiple regulatory signals relaying information about changes in the internal and external environments. Islet cells also need to be made aware about the functional status of neighboring cells through paracrine interactions. All this information is used to orchestrate a hormonal response that contributes to glucose homeostasis. Several neurotransmitters have been proposed to work as paracrine signals in the islet. Most of these, however, have yet to meet the criteria to be considered bona fide paracrine signals, in particular in human islets. Here, we review recent findings describing autocrine and paracrine signaling mechanisms in human islets. These recent results are showing an increasingly complex picture of paracrine interactions in the human islet and emphasize that results from other species cannot be readily extrapolated to the human context. Investigators are unveiling new signaling mechanisms or finding new roles for known paracrine signals in human islets. While it is too early to provide a synthesis, the field of islet research is defining the paracrine and autocrine components that will be used to generate models about how islet function is regulated. Meanwhile, the identified signaling pathways can be proposed as therapeutic targets for treating diabetes, a devastating disease affecting millions worldwide.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures
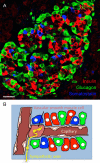



References
-
- Braun M, Ramracheya R, Bengtsson M, Zhang Q, Karanauskaite J, Partridge C, et al. Voltage-gated ion channels in human pancreatic beta-cells: electrophysiological characterization and role in insulin secretion. Diabetes. 2008;57:1618–28. - PubMed
-
- Braun M, Ramracheya R, Amisten S, Bengtsson M, Moritoh Y, Zhang Q, et al. Somatostatin release, electrical activity, membrane currents and exocytosis in human pancreatic delta cells. Diabetologia. 2009 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

