Ascorbic acid efficiently enhances neuronal synthesis of norepinephrine from dopamine
- PMID: 23022576
- PMCID: PMC3527656
- DOI: 10.1016/j.brainresbull.2012.09.009
Ascorbic acid efficiently enhances neuronal synthesis of norepinephrine from dopamine
Abstract
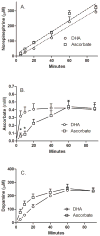
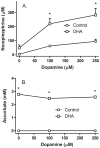
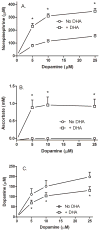
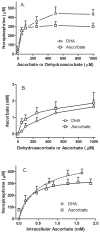
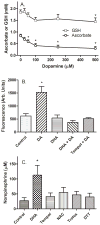
Ascorbic acid enhances synthesis of norepinephrine from dopamine in adrenal chromaffin cells by serving as a co-factor for chromaffin granule dopamine β-hydroxylase (DβH). However, there is controversy regarding in situ kinetics of the ascorbate effect in chromaffin cells, as well as whether they apply to neuronal cells. In this study we evaluated the stimulation of norepinephrine synthesis from dopamine in cultured SH-SY5Y neuroblastoma cells. These cells contained neither ascorbate nor norepinephrine in culture, but when provided with dopamine, they generated intracellular norepinephrine at rates that were stimulated several-fold by intracellular ascorbate. Ascorbate-induced increases in norepinephrine synthesis in dopamine-treated cells were linear over 60 min, despite saturation of intracellular ascorbate. Norepinephrine accumulation after 60 min of incubation with 100 μM dopamine was half-maximal at intracellular ascorbate concentrations of 0.2-0.5 mM, which fits well with the literature K(m) for ascorbate of DβH using dopamine as a substrate. Moreover, these ascorbate concentrations were generated by initial extracellular ascorbate concentrations of less than 25 μM due to concentrative accumulation by the ascorbate transporter. Treatment with 100 μM dopamine acutely increased cellular superoxide generation, which was prevented by ascorbate loading, but associated with a decrease in intracellular ascorbate when the latter was present at concentrations under 1 mM. These results show that ascorbate promptly enhances norepinephrine synthesis from dopamine by neuronal cells that it does so at physiologic intracellular concentrations in accord with the kinetics of DβH, and that it both protects cells from superoxide and by providing electrons to DβH.
Copyright © 2012 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures
References
-
- Mallet J. The TiPS/TINS Lecture. Catecholamines: from gene regulation to neuropsychiatric disorders. Trends Neurosci. 1996;19:191–196. - PubMed
-
- Levin EY, Levenberg B, Kaufman S. The enzymatic conversion of 3,4-dihydroxyphenylethylamine to norepinephrine. J Biol Chem. 1960;235:2080–2086. - PubMed
-
- Diliberto EJ, Jr, Daniels AJ, Viveros OH. Multicompartmental secretion of ascorbate and its dual role in dopamine beta-hydroxylation. Am J Clin Nutr. 1991;54:1163S–1172S. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

