Human epidermal growth factor receptor-2-positive breast cancer: does estrogen receptor status define two distinct subtypes?
- PMID: 23022997
- PMCID: PMC3551479
- DOI: 10.1093/annonc/mds286
Human epidermal growth factor receptor-2-positive breast cancer: does estrogen receptor status define two distinct subtypes?
Abstract
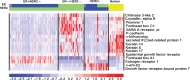
Background: Human epidermal growth factor receptor-2 (HER2) overexpression occurs in ∼20% of breast cancers and has historically been associated with decreased survival. Despite substantial improvements in clinical outcomes, particularly with the emergence of HER2-targeted therapy, a substantial minority of patients still relapses, and progression is inevitable in metastatic disease. Accumulating data indicate that HER2-positive disease is itself a heterogeneous entity.
Methods and results: In this article, we qualitatively review the data supporting the classification of HER2-positive disease as at least two separate entities, distinguished by estrogen receptor (ER) status. We summarize differences in clinical outcomes, including response to neoadjuvant therapy, timing and patterns of dissemination, efficacy of therapy in the metastatic setting and survival outcomes.
Conclusions: The collective data are sufficiently strong at this point to propose that ER status defines two distinct subtypes within HER2-positive breast cancer, and we highlight the implications of this knowledge in future research, including understanding of the basic biology of HER2-positive breast cancer and the design of future clinical trials.
Figures
References
-
- Perou CM, Sorlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. - PubMed
-
- Slamon DJ, Clark GM, Wong SG, et al. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. - PubMed
-
- Konecny G, Pauletti G, Pegram M, et al. Quantitative association between HER-2/neu and steroid hormone receptors in hormone receptor-positive primary breast cancer. J Natl Cancer Inst. 2003;95:142–153. - PubMed
-
- Marsigliante S, Muscella A, Ciardo V, et al. Enzyme-linked immunosorbent assay of HER-2/neu gene product (p185) in breast cancer: its correlation with sex steroid receptors, cathepsin D and histologic grades. Cancer Lett. 1993;75:195–206. - PubMed
-
- Dabbs DJ, Klein ME, Mohsin SK, et al. High false-negative rate of HER2 quantitative reverse transcription polymerase chain reaction of the Oncotype DX test: an independent quality assurance study. J Clin Oncol. 2011;29:4279–4285. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

