Discovery of an allosteric mechanism for the regulation of HCV NS3 protein function
- PMID: 23023261
- PMCID: PMC3480716
- DOI: 10.1038/nchembio.1081
Discovery of an allosteric mechanism for the regulation of HCV NS3 protein function
Abstract
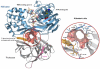
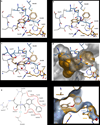
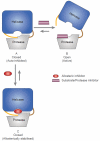
Here we report a highly conserved new binding site located at the interface between the protease and helicase domains of the hepatitis C virus (HCV) NS3 protein. Using a chemical lead, identified by fragment screening and structure-guided design, we demonstrate that this site has a regulatory function on the protease activity via an allosteric mechanism. We propose that compounds binding at this allosteric site inhibit the function of the NS3 protein by stabilizing an inactive conformation and thus represent a new class of direct-acting antiviral agents.
Figures
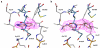
References
-
- Reed KE, Rice CM. Overview of hepatitis c virus genome structure, polyprotein processing and protein properties. Curr. Top. Microbiol. Immunol. 2000;242:55–84. - PubMed
-
- Bartenschlager R, Lohmann V. Replication of hepatitis C virus. J. Gen. Virol. 2000;81:1631–1648. - PubMed
-
- Beran RK, Serebrov V, Pyle AM. The Serine Protease domain of Hepatitis C Viral NS3 Activates RNA Helicase Activity by Promoting the Binding of RNA Substrate. J. Biol. Chem. 2007;282:34913–34920. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- PubChem-Substance/144100018
- PubChem-Substance/144100019
- PubChem-Substance/144100020
- PubChem-Substance/144100021
- PubChem-Substance/144100022
- PubChem-Substance/144100023
- PubChem-Substance/144100024
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information

