Toll-like receptor 7 regulates pancreatic carcinogenesis in mice and humans
- PMID: 23023703
- PMCID: PMC3484447
- DOI: 10.1172/JCI63606
Toll-like receptor 7 regulates pancreatic carcinogenesis in mice and humans
Abstract
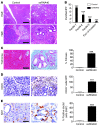
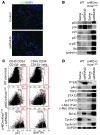
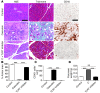
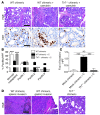
Pancreatic ductal adenocarcinoma is an aggressive cancer that interacts with stromal cells to produce a highly inflammatory tumor microenvironment that promotes tumor growth and invasiveness. The precise interplay between tumor and stroma remains poorly understood. TLRs mediate interactions between environmental stimuli and innate immunity and trigger proinflammatory signaling cascades. Our finding that TLR7 expression is upregulated in both epithelial and stromal compartments in human and murine pancreatic cancer led us to postulate that carcinogenesis is dependent on TLR7 signaling. In a mouse model of pancreatic cancer, TLR7 ligation vigorously accelerated tumor progression and induced loss of expression of PTEN, p16, and cyclin D1 and upregulation of p21, p27, p53, c-Myc, SHPTP1, TGF-β, PPARγ, and cyclin B1. Furthermore, TLR7 ligation induced STAT3 activation and interfaced with Notch as well as canonical NF-κB and MAP kinase pathways, but downregulated expression of Notch target genes. Moreover, blockade of TLR7 protected against carcinogenesis. Since pancreatic tumorigenesis requires stromal expansion, we proposed that TLR7 ligation modulates pancreatic cancer by driving stromal inflammation. Accordingly, we found that mice lacking TLR7 exclusively within their inflammatory cells were protected from neoplasia. These data suggest that targeting TLR7 holds promise for treatment of human pancreatic cancer.
Figures




References
-
- Miyamoto H, Murakami T, Tsuchida K, Sugino H, Miyake H, Tashiro S. Tumor-stroma interaction of human pancreatic cancer: acquired resistance to anticancer drugs and proliferation regulation is dependent on extracellular matrix proteins. Pancreas. 2004;28(1):38–44. doi: 10.1097/00006676-200401000-00006. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

