Chronic overexpression of PNPLA3I148M in mouse liver causes hepatic steatosis
- PMID: 23023705
- PMCID: PMC3484461
- DOI: 10.1172/JCI65179
Chronic overexpression of PNPLA3I148M in mouse liver causes hepatic steatosis
Abstract
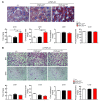
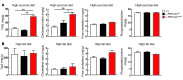
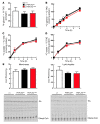
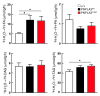
A genetic variant in PNPLA3 (PNPLA3(I148M)), a triacylglycerol (TAG) hydrolase, is a major risk factor for nonalcoholic fatty liver disease (NAFLD); however, the mechanism underlying this association is not known. To develop an animal model of PNPLA3-induced fatty liver disease, we generated transgenic mice that overexpress similar amounts of wild-type PNPLA3 (PNPLA3(WT)) or mutant PNPLA3 (PNPLA3(I148M)) either in liver or adipose tissue. Overexpression of the transgenes in adipose tissue did not affect liver fat content. Expression of PNPLA3(I148M), but not PNPLA3(WT), in liver recapitulated the fatty liver phenotype as well as other metabolic features associated with this allele in humans. Metabolic studies provided evidence for 3 distinct alterations in hepatic TAG metabolism in PNPLA3(I148M) transgenic mice: increased formation of fatty acids and TAG, impaired hydrolysis of TAG, and relative depletion of TAG long-chain polyunsaturated fatty acids. These findings suggest that PNPLA3 plays a role in remodeling TAG in lipid droplets, as they accumulate in response to food intake, and that the increase in hepatic TAG levels associated with the I148M substitution results from multiple changes in hepatic TAG metabolism. The development of an animal model that recapitulates the metabolic phenotype of the allele in humans provides a new platform in which to elucidate the role of PNLPA3(I148M) in NAFLD.
Figures



References
-
- Ludwig J, Viggiano TR, McGill DB, Oh BJ. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin Proc. 1980;55(7):434–438. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

