HEXIM1 controls satellite cell expansion after injury to regulate skeletal muscle regeneration
- PMID: 23023707
- PMCID: PMC3484440
- DOI: 10.1172/JCI62818
HEXIM1 controls satellite cell expansion after injury to regulate skeletal muscle regeneration
Abstract
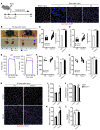
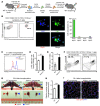
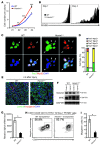
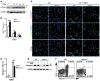
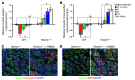
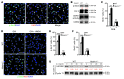
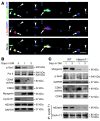
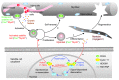
The native capacity of adult skeletal muscles to regenerate is vital to the recovery from physical injuries and dystrophic diseases. Currently, the development of therapeutic interventions has been hindered by the complex regulatory network underlying the process of muscle regeneration. Using a mouse model of skeletal muscle regeneration after injury, we identified hexamethylene bisacetamide inducible 1 (HEXIM1, also referred to as CLP-1), the inhibitory component of the positive transcription elongation factor b (P-TEFb) complex, as a pivotal regulator of skeletal muscle regeneration. Hexim1-haplodeficient muscles exhibited greater mass and preserved function compared with those of WT muscles after injury, as a result of enhanced expansion of satellite cells. Transplanted Hexim1-haplodeficient satellite cells expanded and improved muscle regeneration more effectively than WT satellite cells. Conversely, HEXIM1 overexpression restrained satellite cell proliferation and impeded muscle regeneration. Mechanistically, dissociation of HEXIM1 from P-TEFb and subsequent activation of P-TEFb are required for satellite cell proliferation and the prevention of early myogenic differentiation. These findings suggest a crucial role for the HEXIM1/P-TEFb pathway in the regulation of satellite cell–mediated muscle regeneration and identify HEXIM1 as a potential therapeutic target for degenerative muscular diseases.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

