Lactate and acrylate metabolism by Megasphaera elsdenii under batch and steady-state conditions
- PMID: 23023753
- PMCID: PMC3502912
- DOI: 10.1128/AEM.02443-12
Lactate and acrylate metabolism by Megasphaera elsdenii under batch and steady-state conditions
Abstract
The growth of Megasphaera elsdenii on lactate with acrylate and acrylate analogues was studied under batch and steady-state conditions. Under batch conditions, lactate was converted to acetate and propionate, and acrylate was converted into propionate. Acrylate analogues 2-methyl propenoate and 3-butenoate containing a terminal double bond were similarly converted into their respective saturated acids (isobutyrate and butyrate), while crotonate and lactate analogues 3-hydroxybutyrate and (R)-2-hydroxybutyrate were not metabolized. Under carbon-limited steady-state conditions, lactate was converted to acetate and butyrate with no propionate formed. As the acrylate concentration in the feed was increased, butyrate and hydrogen formation decreased and propionate was increasingly generated, while the calculated ATP yield was unchanged. M. elsdenii metabolism differs substantially under batch and steady-state conditions. The results support the conclusion that propionate is not formed during lactate-limited steady-state growth because of the absence of this substrate to drive the formation of lactyl coenzyme A (CoA) via propionyl-CoA transferase. Acrylate and acrylate analogues are reduced under both batch and steady-state growth conditions after first being converted to thioesters via propionyl-CoA transferase. Our findings demonstrate the central role that CoA transferase activity plays in the utilization of acids by M. elsdenii and allows us to propose a modified acrylate pathway for M. elsdenii.
Figures


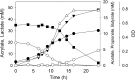

 ) is also shown.
) is also shown.
References
-
- Adams MW. 1990. The structure and mechanism of iron-hydrogenases. Biochim. Biophys. Acta 1020:115–145 - PubMed
-
- Baldwin RL, Wood WA, Emery RS. 1964. Lactate metabolism by Peptostreptococcus elsdenii: evidence for lactyl coenzyme A dehydrase. Biochim. Biophys. Acta 97:202–213 - PubMed
-
- Buckel W, Dorn U, Semmler R. 1981. Glutaconate CoA-transferase from Acidaminococcus fermentans. Eur. J. Biochem. 118:315–321 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

