Stem cell conditioned medium improves acute lung injury in mice: in vivo evidence for stem cell paracrine action
- PMID: 23023971
- PMCID: PMC3532523
- DOI: 10.1152/ajplung.00144.2011
Stem cell conditioned medium improves acute lung injury in mice: in vivo evidence for stem cell paracrine action
Abstract
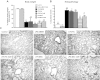
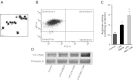
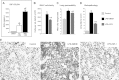
Mortality and morbidity of acute lung injury and acute respiratory distress syndrome remain high because of the lack of pharmacological therapies to prevent injury or promote repair. Mesenchymal stem cells (MSCs) prevent lung injury in various experimental models, despite a low proportion of donor-derived cell engraftment, suggesting that MSCs exert their beneficial effects via paracrine mechanisms. We hypothesized that soluble factors secreted by MSCs promote the resolution of lung injury in part by modulating alveolar macrophage (AM) function. We tested the therapeutic effect of MSC-derived conditioned medium (CdM) compared with whole MSCs, lung fibroblasts, and fibroblast-CdM. Intratracheal MSCs and MSC-CdM significantly attenuated lipopolysaccharide (LPS)-induced lung neutrophil influx, lung edema, and lung injury as assessed by an established lung injury score. MSC-CdM increased arginase-1 activity and Ym1 expression in LPS-exposed AMs. In vivo, AMs from LPS-MSC and LPS-MSC CdM lungs had enhanced expression of Ym1 and decreased expression of inducible nitric oxide synthase compared with untreated LPS mice. This suggests that MSC-CdM promotes alternative macrophage activation to an M2 "healer" phenotype. Comparative multiplex analysis of MSC- and fibroblast-CdM demonstrated that MSC-CdM contained several factors that may confer therapeutic benefit, including insulin-like growth factor I (IGF-I). Recombinant IGF-I partially reproduced the lung protective effect of MSC-CdM. In summary, MSCs act through a paracrine activity. MSC-CdM promotes the resolution of LPS-induced lung injury by attenuating lung inflammation and promoting a wound healing/anti-inflammatory M2 macrophage phenotype in part via IGF-I.
Figures





References
-
- Acquistapace A, Bru T, Lesault PF, Figeac F, Coudert AE, le Coz O, Christov C, Baudin X, Auber F, Yiou R, Dubois-Randé JL, Rodriguez AM. Human mesenchymal stem cells reprogram adult cardiomyocytes toward a progenitor-like state through partial cell fusion and mitochondria transfer. Stem Cells 29: 812–824, 2011 - PMC - PubMed
-
- Amariglio N, Hirshberg A, Scheithauer BW, Cohen Y, Loewenthal R, Trakhtenbrot L, Paz N, Koren-Michowitz M, Waldman D, Leider-Trejo L, Toren A, Constantini S, Rechavi G. Donor-derived brain tumor following neural stem cell transplantation in an ataxia telangiectasia patient. PLoS Med 6: e1000029, 2009 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

