Increased surfactant protein D fails to improve bacterial clearance and inflammation in serpinB1-/- mice
- PMID: 23024061
- PMCID: PMC3547094
- DOI: 10.1165/rcmb.2012-0145OC
Increased surfactant protein D fails to improve bacterial clearance and inflammation in serpinB1-/- mice
Abstract
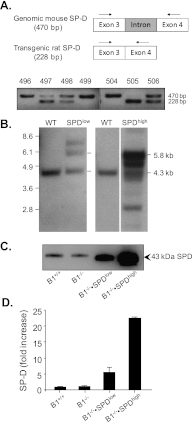
Previously, we described the protective role of the neutrophil serine protease inhibitor serpinB1 in preventing early mortality of Pseudomonas aeruginosa lung infection by fostering bacterial clearance and limiting inflammatory cytokines and proteolytic damage. Surfactant protein D (SP-D), which maintains the antiinflammatory pulmonary environment and mediates bacterial removal, was degraded in infected serpinB1-deficient mice. Based on the hypothesis that increased SP-D would rescue or mitigate the pathological effects of serpinB1 deletion, we generated two serpinB1(-/-) lines overexpressing lung-specific rat SP-D and inoculated the mice with P. aeruginosa. Contrary to predictions, bacterial counts in the lungs of SP-D(low)serpinB1(-/-) and SP-D(high) serpinB1(-/-) mice were 4 logs higher than wild-type and not different from serpinB1(-/-) mice. SP-D overexpression also failed to mitigate inflammation (TNF-α), lung injury (free protein, albumin), or excess neutrophil death (free myeloperoxidase, elastase). These pathological markers were higher for infected SP-D(high)serpinB1(-/-) mice than for serpinB1(-/-) mice, although the differences were not significant after controlling for multiple comparisons. The failure of transgenic SP-D to rescue antibacterial defense of serpinB1-deficient mice occurred despite 5-fold or 20-fold increased expression levels, largely normal structure, and dose-dependent bacteria-aggregating activity. SP-D of infected wild-type mice was intact in 43-kD monomers by reducing SDS-PAGE. By contrast, proteolytic fragments of 35, 17, and 8 kD were found in infected SP-D(low)serpinB1(-/-), SP-D(high) serpinB1(-/-) mice, and serpinB1(-/-) mice. Thus, although therapies to increase lung concentration of SP-D may have beneficial applications, the findings suggest that therapy with SP-D may not be beneficial for lung inflammation or infection if the underlying clinical condition includes excess proteolysis.
Figures


References
-
- Cooley J, Takayama TK, Shapiro SD, Schechter NM, Remold-O’Donnell E. The serpin MNEI inhibits elastase-like and chymotrypsin-like serine proteases through efficient reactions at two active sites. Biochemistry 2001;40:15762–15770 - PubMed
-
- Belaaouaj A, Kim KS, Shapiro SD. Degradation of outer membrane protein A in Escherichia coli killing by neutrophil elastase. Science 2000;289:1185–1188 - PubMed
-
- Reeves EP, Lu H, Jacobs HL, Messina CG, Bolsover S, Gabella G, Potma EO, Warley A, Roes J, Segal AW. Killing activity of neutrophils is mediated through activation of proteases by K+ flux. Nature 2002;416:291–297 - PubMed
-
- Moraes TJ, Zurawska JH, Downey GP. Neutrophil granule contents in the pathogenesis of lung injury. Curr Opin Hematol 2006;13:21–27 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

