Systematic review and network meta-analysis of overall survival comparing 3 mg/kg ipilimumab with alternative therapies in the management of pretreated patients with unresectable stage III or IV melanoma
- PMID: 23024154
- PMCID: PMC3500357
- DOI: 10.1634/theoncologist.2011-0427
Systematic review and network meta-analysis of overall survival comparing 3 mg/kg ipilimumab with alternative therapies in the management of pretreated patients with unresectable stage III or IV melanoma
Abstract
Objective: To compare the overall survival (OS) of patients treated with 3 mg/kg ipilimumab versus alternative systemic therapies in pretreated unresectable stage III or IV melanoma patients.
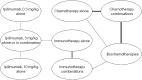
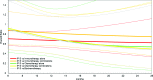
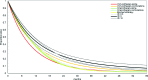
Methods: A systematic literature search was performed to identify relevant randomized clinical trials. From these trials, Kaplan-Meier survival curves for each intervention were digitized and combined by means of a Bayesian network meta-analysis (NMA) to compare different drug classes.
Results: Of 38 trials identified, 15 formed one interlinked network by drug class to allow for an NMA. Ipilimumab, at a dose of 3 mg/kg, was associated with a greater mean OS time (18.8 months; 95% credible interval [CrI], 15.5-23.0 months) than single-agent chemotherapy (12.3 months; 95% CrI, 6.3-28.0 months), chemotherapy combinations (12.2 months; 95% CrI, 7.1-23.3 months), biochemotherapies (11.9 months; 95% CrI, 7.0-22.0 months), single-agent immunotherapy (11.1 months; 95% CrI, 8.5-16.2 months), and immunotherapy combinations (14.1 months; 95% CrI, 9.0-23.8 months).
Conclusion: Results of this NMA were in line with previous findings and suggest that OS with ipilimumab is expected to be greater than with alternative systemic therapies, alone or in combination, for the management of pretreated patients with unresectable stage III or IV melanoma.
Conflict of interest statement
Figures

References
-
- World Health Organization. Skin Cancers - How Common Is Skin Cancer? [accessed August 10, 2011]. Available at http://www.who.int/uv/faq/skincancer/en/index1.html.
-
- Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. - PubMed
-
- Agarwala SS. Current systemic therapy for metastatic melanoma. Expert Rev Anticancer Ther. 2009;9:587–595. - PubMed
-
- Bedikian AY, Millward M, Pehamberger H, et al. Bcl-2 antisense (oblimersen sodium) plus dacarbazine in patients with advanced melanoma: The Oblimersen Melanoma Study Group. J Clin Oncol. 2006;24:4738–4745. - PubMed
-
- Tsao H, Atkins MB, Sober AJ. Management of cutaneous melanoma. N Engl J Med. 2004;351:998–1012. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

