Molecular parallels between neural and vascular development
- PMID: 23024177
- PMCID: PMC3530036
- DOI: 10.1101/cshperspect.a006551
Molecular parallels between neural and vascular development
Abstract
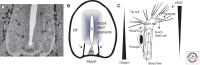
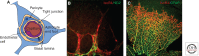
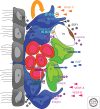
The human central nervous system (CNS) features a network of ~400 miles of blood vessels that receives >20% of the body's cardiac output and uses most of its blood glucose. Many human diseases, including stroke, retinopathy, and cancer, are associated with the biology of CNS blood vessels. These vessels originate from extrinsic cell populations, including endothelial cells and pericytes that colonize the CNS and interact with glia and neurons to establish the blood-brain barrier and control cerebrovascular exchanges. Neurovascular interactions also play important roles in adult neurogenic niches, which harbor a unique population of neural stem cells that are intimately associated with blood vessels. We here review the cellular and molecular mechanisms required to establish the CNS vascular network, with a special focus on neurovascular interactions and the functions of vascular endothelial growth factors.
Figures
References
-
- Andreu-Agulló C, Morante-Redolat JM, Delgado AC, Fariñas I 2009. Vascular niche factor PEDF modulates Notch-dependent stemness in the adult subependymal zone. Nat Neurosci 12: 1514–1523 - PubMed
-
- Armulik A, Genové G, Mäe M, Nisancioglu MH, Wallgard E, Niaudet C, He L, Norlin J, Lindblom P, Strittmatter K, et al. 2010. Pericytes regulate the blood–brain barrier. Nature 468: 557–561 - PubMed
-
- Bellon A, Luchino J, Haigh K, Rougon G, Haigh J, Chauvet S, Mann F 2010. VEGFR2 (KDR/Flk1) signaling mediates axon growth in response to semaphorin 3E in the developing brain. Neuron 66: 205–219 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
