Insights into dietary flavonoids as molecular templates for the design of anti-platelet drugs
- PMID: 23024269
- PMCID: PMC3527766
- DOI: 10.1093/cvr/cvs304
Insights into dietary flavonoids as molecular templates for the design of anti-platelet drugs
Abstract
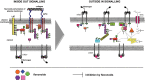
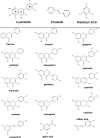
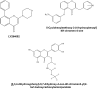
Flavonoids are low-molecular weight, aromatic compounds derived from fruits, vegetables, and other plant components. The consumption of these phytochemicals has been reported to be associated with reduced cardiovascular disease (CVD) risk, attributed to their anti-inflammatory, anti-proliferative, and anti-thrombotic actions. Flavonoids exert these effects by a number of mechanisms which include attenuation of kinase activity mediated at the cell-receptor level and/or within cells, and are characterized as broad-spectrum kinase inhibitors. Therefore, flavonoid therapy for CVD is potentially complex; the use of these compounds as molecular templates for the design of selective and potent small-molecule inhibitors may be a simpler approach to treat this condition. Flavonoids as templates for drug design are, however, poorly exploited despite the development of analogues based on the flavonol, isoflavonone, and isoflavanone subgroups. Further exploitation of this family of compounds is warranted due to a structural diversity that presents great scope for creating novel kinase inhibitors. The use of computational methodologies to define the flavonoid pharmacophore together with biological investigations of their effects on kinase activity, in appropriate cellular systems, is the current approach to characterize key structural features that will inform drug design. This focussed review highlights the potential of flavonoids to guide the design of clinically safer, more selective, and potent small-molecule inhibitors of cell signalling, applicable to anti-platelet therapy.
Figures
Similar articles
-
Investigating flavonoids as molecular templates for the design of small-molecule inhibitors of cell signaling.J Food Sci. 2013 Dec;78(12):N1921-8. doi: 10.1111/1750-3841.12293. J Food Sci. 2013. PMID: 24329957
-
GRID and docking analyses reveal a molecular basis for flavonoid inhibition of Src family kinase activity.J Nutr Biochem. 2015 Nov;26(11):1156-65. doi: 10.1016/j.jnutbio.2015.05.004. Epub 2015 Jun 9. J Nutr Biochem. 2015. PMID: 26140983
-
A structural basis for the inhibition of collagen-stimulated platelet function by quercetin and structurally related flavonoids.Br J Pharmacol. 2010 Mar;159(6):1312-25. doi: 10.1111/j.1476-5381.2009.00632.x. Epub 2010 Feb 10. Br J Pharmacol. 2010. PMID: 20148891 Free PMC article.
-
A review of in vitro studies of the anti-platelet potential of citrus fruit flavonoids.Food Chem Toxicol. 2021 Apr;150:112090. doi: 10.1016/j.fct.2021.112090. Epub 2021 Feb 23. Food Chem Toxicol. 2021. PMID: 33636212 Review.
-
Platelet Signaling Pathways and New Inhibitors.Arterioscler Thromb Vasc Biol. 2018 Apr;38(4):e28-e35. doi: 10.1161/ATVBAHA.118.310224. Arterioscler Thromb Vasc Biol. 2018. PMID: 29563117 Free PMC article. Review. No abstract available.
Cited by
-
Identification of plant-derived natural products as potential inhibitors of the Mycobacterium tuberculosis proteasome.BMC Complement Altern Med. 2014 Oct 15;14:400. doi: 10.1186/1472-6882-14-400. BMC Complement Altern Med. 2014. PMID: 25315519 Free PMC article.
-
Inhibition of Inositol Polyphosphate Kinases by Quercetin and Related Flavonoids: A Structure-Activity Analysis.J Med Chem. 2019 Feb 14;62(3):1443-1454. doi: 10.1021/acs.jmedchem.8b01593. Epub 2019 Jan 25. J Med Chem. 2019. PMID: 30624931 Free PMC article.
-
Pharmacological actions of nobiletin in the modulation of platelet function.Br J Pharmacol. 2015 Aug;172(16):4133-45. doi: 10.1111/bph.13191. Epub 2015 Jun 26. Br J Pharmacol. 2015. PMID: 25988959 Free PMC article.
-
Drug Response Diversity: A Hidden Bacterium?J Pers Med. 2021 Apr 25;11(5):345. doi: 10.3390/jpm11050345. J Pers Med. 2021. PMID: 33922920 Free PMC article. Review.
-
Epigallocatechin gallate inhibits release of extracellular vesicles from platelets without inhibiting phosphatidylserine exposure.Sci Rep. 2021 Sep 3;11(1):17678. doi: 10.1038/s41598-021-97212-8. Sci Rep. 2021. PMID: 34480042 Free PMC article.
References
-
- Hertog MGL, Hollman PCH, Katan MB. Content of potentially anticarcinogenic flavonoids of 28 vegetables and 9 fruits commonly consumed in the Netherlands. J Agric Food Chem. 1992;40:2379–2383. doi:10.1021/jf00024a011. - DOI
-
- Justesen U, Knuthsen P, Leth T. Quantitative analysis of flavonols, flavones, and flavanones in fruits, vegetables and beverages by high-performance liquid chromatography with photo-diode array and mass spectrometric detection. J Chromatogr A. 1998;799:101–110. doi:10.1016/S0021-9673(97)01061-3. - DOI - PubMed
-
- Hertog MGL, Feskens EJM, Hollman PCH, Katan MB, Kromhout D. Dietary antioxidant flavonoids and risk of coronary heart disease: the Zutphen Elderly Study. The Lancet. 1993;342:1007–1011. doi:10.1016/0140-6736(93)92876-U. - DOI - PubMed
-
- Crozier A, Lean MEJ, McDonald MS, Black C. Quantitative analysis of the flavonoid content of commercial tomatoes, onions, lettuce, and celery. J Agric Food Chem. 1997;45:590–595. doi:10.1021/jf960339y. - DOI
-
- Macheix JJ, Fleuriet A, Billot J. Fruit Phenolics. Boca Raton, FL: CRC Press; 1990.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

