A universal method for the identification of bacteria based on general PCR primers
- PMID: 23024404
- PMCID: PMC3209952
- DOI: 10.1007/s12088-011-0122-5
A universal method for the identification of bacteria based on general PCR primers
Abstract
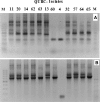
The Universal Method (UM) described here will allow the detection of any bacterial rDNA leading to the identification of that bacterium. The method should allow prompt and accurate identification of bacteria. The principle of the method is simple; when a pure PCR product of the 16S gene is obtained, sequenced, and aligned against bacterial DNA data base, then the bacterium can be identified. Confirmation of identity may follow. In this work, several general 16S primers were designed, mixed and applied successfully against 101 different bacterial isolates. One mixture, the Golden mixture7 (G7) detected all tested isolates (67/67). Other golden mixtures; G11, G10, G12, and G5 were useful as well. The overall sensitivity of the UM was 100% since all 101 isolates were detected yielding intended PCR amplicons. A selected PCR band from each of 40 isolates was sequenced and the bacterium identified to species or genus level using BLAST. The results of the UM were consistent with bacterial identities as validated with other identification methods; cultural, API 20E, API 20NE, or genera and species specific PCR primers. Bacteria identified in the study, covered 34 species distributed among 24 genera. The UM should allow the identification of species, genus, novel species or genera, variations within species, and detection of bacterial DNA in otherwise sterile samples such as blood, cerebrospinal fluid, manufactured products, medical supplies, cosmetics, and other samples. Applicability of the method to identifying members of bacterial communities is discussed. The approach itself can be applied to other taxa such as protists and nematodes.
Keywords: Bacterial identification; Golden mixture; Multiplex; Universal method; Universal primers.
Figures






References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
