PDE5 Inhibitors as Potential Tools in the Treatment of Cystic Fibrosis
- PMID: 23024633
- PMCID: PMC3444771
- DOI: 10.3389/fphar.2012.00167
PDE5 Inhibitors as Potential Tools in the Treatment of Cystic Fibrosis
Abstract
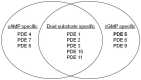
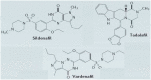
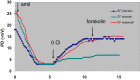
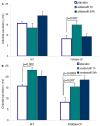
Despite great advances in the understanding of the genetics and pathophysiology of cystic fibrosis (CF), there is still no cure for the disease. Using phosphodiesterase type 5 (PDE5) inhibitors, we and others have provided evidence of rescued F508del-CFTR trafficking and corrected deficient chloride transport activity. Studies using PDE5 inhibitors in mice homozygous for the clinically relevant F508del mutation have been conducted with the aim of restoring F508del-CFTR protein function. We demonstrated, by measuring transepithelial nasal potential difference in F508del mice following intraperitoneal injection of sildenafil, vardenafil, or taladafil at clinical doses are able to restore the decreased CFTR-dependent chloride transport across the nasal mucosa. Moreover, vardenafil, but not sildenafil, stimulates chloride transport through the normal CFTR protein. We developed a specific nebulizer setup for mice, with which we demonstrated, through a single inhalation of PDE5 inhibitors, local activation of CFTR protein in CF. Significant potential advantages of inhalation drug therapy over oral or intravenous routes include rapid onset of pharmacological action, reduced systemic secondary effects, and reduced effective drug doses compared to the drug delivered orally; this underlines the relevance and impact of our work for translational science. More recently, we analyzed the bronchoalveolar lavage of CF and wild-type mice for cell infiltrates and expression of pro-inflammatory cytokines and chemokines; we found that the CFTR activating effect of vardenafil, selected as a representative long-lasting PDE5 inhibitor, breaks the vicious circle of lung inflammation which plays a major role in morbi-mortality in CF. Our data highlight the potential use of PDE5 inhibitors in CF. Therapeutic approaches using clinically approved PDE5 inhibitors to address F508del-CFTR defects could speed up the development of new therapies for CF.
Keywords: CFTR; PDE5 inhibitors; cystic fibrosis; sildenafil; taladafil; vardenafil.
Figures

Similar articles
-
Correction of chloride transport and mislocalization of CFTR protein by vardenafil in the gastrointestinal tract of cystic fibrosis mice.PLoS One. 2013 Oct 24;8(10):e77314. doi: 10.1371/journal.pone.0077314. eCollection 2013. PLoS One. 2013. PMID: 24204804 Free PMC article.
-
Inhaled phosphodiesterase type 5 inhibitors restore chloride transport in cystic fibrosis mice.Eur Respir J. 2011 Jan;37(1):72-8. doi: 10.1183/09031936.00013510. Epub 2010 Jun 18. Eur Respir J. 2011. PMID: 20562123
-
Vardenafil reduces macrophage pro-inflammatory overresponses in cystic fibrosis through PDE5- and CFTR-dependent mechanisms.Clin Sci (Lond). 2017 Jun 1;131(11):1107-1121. doi: 10.1042/CS20160749. Epub 2017 Feb 14. Clin Sci (Lond). 2017. PMID: 28196856
-
Manipulating proteostasis to repair the F508del-CFTR defect in cystic fibrosis.Mol Cell Pediatr. 2016 Dec;3(1):13. doi: 10.1186/s40348-016-0040-z. Epub 2016 Mar 14. Mol Cell Pediatr. 2016. PMID: 26976279 Free PMC article. Review.
-
Efficacy and Safety of CFTR Corrector and Potentiator Combination Therapy in Patients with Cystic Fibrosis for the F508del-CFTR Homozygous Mutation: A Systematic Review and Meta-analysis.Adv Ther. 2019 Feb;36(2):451-461. doi: 10.1007/s12325-018-0860-4. Epub 2018 Dec 15. Adv Ther. 2019. PMID: 30554331
Cited by
-
Optical Nanosensors for in vivo Physiological Chloride Detection for Monitoring Cystic Fibrosis Treatment.Anal Methods. 2020 Mar 21;12(11):1441-1448. doi: 10.1039/C9AY02717C. Epub 2020 Feb 26. Anal Methods. 2020. PMID: 32226484 Free PMC article.
-
Pulmonary hypertension survival effects and treatment options in cystic fibrosis.Curr Opin Pulm Med. 2013 Nov;19(6):652-61. doi: 10.1097/MCP.0b013e3283659e9f. Curr Opin Pulm Med. 2013. PMID: 24048083 Free PMC article. Review.
-
Cyclic AMP and glycogen synthase kinase 3 form a regulatory loop in spermatozoa.J Cell Physiol. 2018 Sep;233(9):7239-7252. doi: 10.1002/jcp.26557. Epub 2018 Mar 25. J Cell Physiol. 2018. PMID: 29574946 Free PMC article.
-
Nitric Oxide System and Bronchial Epithelium: More Than a Barrier.Front Physiol. 2021 Jun 30;12:687381. doi: 10.3389/fphys.2021.687381. eCollection 2021. Front Physiol. 2021. PMID: 34276407 Free PMC article. Review.
-
New and Emerging Treatments for Cystic Fibrosis.Drugs. 2015 Jul;75(11):1165-75. doi: 10.1007/s40265-015-0424-8. Drugs. 2015. PMID: 26091951 Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

