DUEXIS(®) (ibuprofen 800 mg, famotidine 26.6 mg): a new approach to gastroprotection for patients with chronic pain and inflammation who require treatment with a nonsteroidal anti-inflammatory drug
- PMID: 23024710
- PMCID: PMC3458616
- DOI: 10.1177/1759720X12444710
DUEXIS(®) (ibuprofen 800 mg, famotidine 26.6 mg): a new approach to gastroprotection for patients with chronic pain and inflammation who require treatment with a nonsteroidal anti-inflammatory drug
Abstract
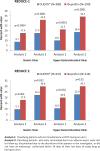
Chronic pain conditions affect at least 116 million US adults and more than one-third of adults worldwide. Nonsteroidal anti-inflammatory drugs (NSAIDs) are used extensively for the treatment of chronic pain due to their efficacy as anti-inflammatory and analgesic agents. Gastrointestinal toxicity is the most well known adverse effect of NSAID therapy and it may manifest as dyspepsia, ulcers, or bleeding. Current guidelines for the management of patients who require NSAIDs for chronic pain and inflammation recognize the potential toxicity associated with these drugs and the need for gastroprotection. DUEXIS(®) (ibuprofen 800 mg, famotidine 26.6 mg) is a proprietary combination, immediate release tablet containing 800 mg of ibuprofen and 26.6 mg of famotidine. The efficacy of DUEXIS(®) taken three times daily has been demonstrated in two large-scale controlled clinical trials (Registration Endoscopic Studies to Determine Ulcer Formation of HZT-501 Compared with Ibuprofen: Efficacy and Safety Studies (REDUCE) and REDUCE-2) which showed that this new formulation significantly reduced the risk of endoscopic upper gastrointestinal ulcers compared with ibuprofen alone (REDUCE-1, p < 0.0001, REDUCE-2, p <0.05). DUEXIS(®) was also superior to ibuprofen in decreasing the risk for gastric ulcers (REDUCE-1, p < 0.001, REDUCE-2, p < 0.05) as well as duodenal ulcers (REDUCE-1, p < 0.05, REDUCE-2, p < 0.05). Safety results from these two studies indicated that treatment-emergent adverse events occurred in 55% of patients treated with DUEXIS(®)versus 58.7% for ibuprofen, and serious adverse events were recorded for 3.2% of patients treated with DUEXIS(®)versus 3.3% of those on ibuprofen. Adverse events leading to discontinuation occurred in 6.7% of patients treated with DUEXIS(®) and 7.6% for ibuprofen. The combination of ibuprofen and famotidine in a single tablet has the potential to improve adherence to gastroprotective therapy in patients who require NSAID treatment and the use of a histamine type 2 receptor antagonist rather than a proton-pump inhibitor may decrease the risk for clinically significant drug interactions and adverse events (e.g. interaction with clopidogrel, fracture, pneumonia, Clostridium difficile infection).
Keywords: chronic pain; famotidine; gastrointestinal; ibuprofen; proton-pump inhibitor; ulcer.
Figures

Similar articles
-
Fixed-dose ibuprofen/famotidine: a review of its use to reduce the risk of gastric and duodenal ulcers in patients requiring NSAID therapy.Clin Drug Investig. 2013 Sep;33(9):689-97. doi: 10.1007/s40261-013-0113-x. Clin Drug Investig. 2013. PMID: 23881568 Review.
-
HZT-501 (DUEXIS(®); ibuprofen 800 mg/famotidine 26.6 mg) gastrointestinal protection in the treatment of the signs and symptoms of rheumatoid arthritis and osteoarthritis.Expert Rev Gastroenterol Hepatol. 2012 Feb;6(1):25-35. doi: 10.1586/egh.11.88. Expert Rev Gastroenterol Hepatol. 2012. PMID: 22149579 Review.
-
A fixed-dose combination ibuprofen and famotidine (Duexis).Med Lett Drugs Ther. 2011 Oct 31;53(1376):85-6. Med Lett Drugs Ther. 2011. PMID: 22033211
-
Double-blind randomized trials of single-tablet ibuprofen/high-dose famotidine vs. ibuprofen alone for reduction of gastric and duodenal ulcers.Am J Gastroenterol. 2012 Mar;107(3):379-86. doi: 10.1038/ajg.2011.443. Epub 2011 Dec 20. Am J Gastroenterol. 2012. PMID: 22186979 Free PMC article. Clinical Trial.
-
Risk of upper gastrointestinal ulcers in patients with osteoarthritis receiving single-tablet ibuprofen/famotidine versus ibuprofen alone: pooled efficacy and safety analyses of two randomized, double-blind, comparison trials.Postgrad Med. 2014 Jul;126(4):82-91. doi: 10.3810/pgm.2014.07.2786. Postgrad Med. 2014. PMID: 25141246
Cited by
-
Rheumatiod Arthritis: An Updated Overview of Latest Therapy and Drug Delivery.J Pharmacopuncture. 2019 Dec;22(4):210-224. doi: 10.3831/KPI.2019.22.029. Epub 2019 Dec 31. J Pharmacopuncture. 2019. PMID: 31970018 Free PMC article. Review.
-
Fixed-dose ibuprofen/famotidine: a review of its use to reduce the risk of gastric and duodenal ulcers in patients requiring NSAID therapy.Clin Drug Investig. 2013 Sep;33(9):689-97. doi: 10.1007/s40261-013-0113-x. Clin Drug Investig. 2013. PMID: 23881568 Review.
-
Gastrointestinal injury associated with NSAID use: a case study and review of risk factors and preventative strategies.Drug Healthc Patient Saf. 2015 Jan 22;7:31-41. doi: 10.2147/DHPS.S71976. eCollection 2015. Drug Healthc Patient Saf. 2015. PMID: 25653559 Free PMC article. Review.
-
Management of Osteoarthritis with Avocado/Soybean Unsaponifiables.Cartilage. 2015 Jan;6(1):30-44. doi: 10.1177/1947603514554992. Cartilage. 2015. PMID: 25621100 Free PMC article.
-
Merging konjac glucomannan with other copolymeric hydrogels as a cutting-edge liquid raft system for dual delivery of etoricoxib and famotidine.Drug Deliv. 2023 Dec;30(1):2189630. doi: 10.1080/10717544.2023.2189630. Drug Deliv. 2023. PMID: 36927148 Free PMC article.
References
-
- Aabakken L., Bjørnbeth B.A., Weberg R., Viksmoen L., Larsen S., Osnes M. (1990) NSAID-associated gastroduodenal damage: does famotidine protection extend into the mid and distal duodenum? Alimentary Pharmacol Ther 4: 295–303 - PubMed
-
- Abdrabbo M.K., Puera D.A., Wilcox C.M. (2004) NSAID-associated GI complications: available management options and identification of high-risk patients. Gastro Endo News 2: 65–68
-
- Abraham N.S., El-Serag H.B., Johnson M.L., Hartman C., Richardson R., Ray W.A., et al. (2005) National adherence to evidence-based guidelines for the prescription of nonsteroidal antiinflammatory drugs. Gastroenterology 129: 1171–1178 - PubMed
-
- Arthrotec prescribing information (2010) Pfizer: New York, NY; Available at: http://labeling.pfizer.com/ShowLabeling.aspx?id=526 (accessed 10 April 2012).
-
- Bangalore S., Kamalakkannan G., Parkar S., Messerli F.H. (2007) Fixed-dose combinations improve medication compliance: a meta-analysis. Am J Med 120: 713–719 - PubMed
LinkOut - more resources
Full Text Sources

