The Role of Magnesium Supplementation in Cisplatin-induced Nephrotoxicity in a Rat Model: No Nephroprotectant Effect
- PMID: 23024853
- PMCID: PMC3445280
The Role of Magnesium Supplementation in Cisplatin-induced Nephrotoxicity in a Rat Model: No Nephroprotectant Effect
Abstract
Objectives: Cisplatin (CP) is used as the commonest drug to treat solid tumors. It is accompanied by a nephrotoxicity side effect. The main objective of this study is to investigate the protective role of magnesium (Mg) supplementation in CP-induced nephrotoxicity in a rat model.
Methods: Twenty-nine Wistar rats were randomly assigned to four groups (1-4). Groups 1-3 received 20, 80, and 200 mg/kg magnesium sulfate respectively, for 10 days, but on day 3, a single dose of CP (7 mg/kg, i.p.) was also injected. Group 4 (positive control group) received the same regimen of Groups 1-3 except saline instead magnesium sulfate. One week after CP administration, blood samples were obtained and all animals were killed for kidney histopathological investigations.
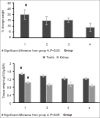
Results: All CP-treated animals lost weight, and the percentage of weight loss in Group 1 (low dose Mg sulfate treated) was significantly higher compared with the positive control group (Group 4, P < 0.05). The increase in blood urea nitrogen (BUN) and creatinine (Cr) levels in serum in Group 1 were more than those in other groups (P < 0.05). No statistical differences were observed in serum magnesium, nitrite, and total protein levels among the groups. The kidney tissue damage in Groups 1-3 was not significantly different when compared with Group 4. Moreover, the kidney and testis weights in Group 1 were significantly greater than those in the positive control group (P < 0.05).
Conclusion: Regarding the BUN and Cr levels in the serum, kidneys weight, and the histopathological study, the low dose of Mg supplementation intensifies kidney toxicity and renal dysfunction in CP-induced nephrotoxicity in the rat model. However, the protective role of Mg with moderate and high doses is not certain.
Keywords: Cisplatin; magnesium; nephrotoxicity; rat.
Conflict of interest statement
Figures


References
-
- Lee YC, Saijo N, Sasaki Y, Takahashi H, Sakurai M, Ishihara J, et al. Antitumor effect of two-drug simultaneous or sequential use of cisplatin, vindesine or etoposide on human pulmonary adenocarcinoma cell lines in tumor clonogenic assay. Jpn J Cancer Res. 1986;77:312–8. - PubMed
-
- Pinto AL, Lippard SJ. Binding of the antitumor drug cis-diamminedichloroplatinum(II) (cisplatin) to DNA. Biochim Biophys Acta. 1985;780:167–80. - PubMed
-
- Shord SS, Thompson DM, Krempl GA, Hanigan MH. Effect of concurrent medications on cisplatin-induced nephrotoxicity in patients with head and neck cancer. Anticancer Drugs. 2006;17:207–15. - PubMed
-
- Erdlenbruch B, Nier M, Kern W, Hiddemann W, Pekrun A, Lakomek M. Pharmacokinetics of cisplatin and relation to nephrotoxicity in paediatric patients. Eur J Clin Pharmacol. 2001;57:393–402. - PubMed
-
- Nagai N, Kinoshita M, Ogata H, Tsujino D, Wada Y, Someya K, et al. Relationship between pharmacokinetics of unchanged cisplatin and nephrotoxicity after intravenous infusions of cisplatin to cancer patients. Cancer Chemother Pharmacol. 1996;39:131–7. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
