Addressing statistical biases in nucleotide-derived protein databases for proteogenomic search strategies
- PMID: 23025403
- PMCID: PMC3703792
- DOI: 10.1021/pr300411q
Addressing statistical biases in nucleotide-derived protein databases for proteogenomic search strategies
Abstract
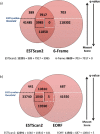
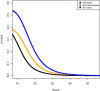
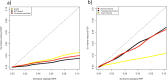
Proteogenomics has the potential to advance genome annotation through high quality peptide identifications derived from mass spectrometry experiments, which demonstrate a given gene or isoform is expressed and translated at the protein level. This can advance our understanding of genome function, discovering novel genes and gene structure that have not yet been identified or validated. Because of the high-throughput shotgun nature of most proteomics experiments, it is essential to carefully control for false positives and prevent any potential misannotation. A number of statistical procedures to deal with this are in wide use in proteomics, calculating false discovery rate (FDR) and posterior error probability (PEP) values for groups and individual peptide spectrum matches (PSMs). These methods control for multiple testing and exploit decoy databases to estimate statistical significance. Here, we show that database choice has a major effect on these confidence estimates leading to significant differences in the number of PSMs reported. We note that standard target:decoy approaches using six-frame translations of nucleotide sequences, such as assembled transcriptome data, apparently underestimate the confidence assigned to the PSMs. The source of this error stems from the inflated and unusual nature of the six-frame database, where for every target sequence there exists five "incorrect" targets that are unlikely to code for protein. The attendant FDR and PEP estimates lead to fewer accepted PSMs at fixed thresholds, and we show that this effect is a product of the database and statistical modeling and not the search engine. A variety of approaches to limit database size and remove noncoding target sequences are examined and discussed in terms of the altered statistical estimates generated and PSMs reported. These results are of importance to groups carrying out proteogenomics, aiming to maximize the validation and discovery of gene structure in sequenced genomes, while still controlling for false positives.
Figures



References
-
- Schrimpf S. P.; Weiss M.; Reiter L.; Ahrens C. H.; Jovanovic M.; Malmstroem J.; Brunner E.; Mohanty S.; Lercher M. J.; Hunziker P. E.; Aebersold R.; von Mering C.; Hengartner M. O. Comparative functional analysis of the Caenorhabditis elegans and Drosophila melanogaster proteomes. PLoS Biol. 2009, 7(3), e48. - PMC - PubMed
-
- Chaerkady R.; Kelkar D. S.; Muthusamy B.; Kandasamy K.; Dwivedi S. B.; Sahasrabuddhe N. A.; Kim M. S.; Renuse S.; Pinto S. M.; Sharma R.; Pawar H.; Sekhar N. R.; Mohanty A. K.; Getnet D.; Yang Y.; Zhong J.; Dash A. P.; Maccallum R. M.; Delanghe B.; Mlambo G.; Kumar A.; Keshava Prasad T. S.; Okulate M.; Kumar N.; Pandey A. A proteogenomic analysis of Anopheles gambiae using high-resolution Fourier transform mass spectrometry. Genome Res. 2011, 21(11), 1872–1881. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

