Pharmacokinetics of low-dose cidofovir in kidney transplant recipients with BK virus infection
- PMID: 23025519
- PMCID: PMC3654813
- DOI: 10.1111/tid.12014
Pharmacokinetics of low-dose cidofovir in kidney transplant recipients with BK virus infection
Abstract
Background: BK virus (BKV) infection in kidney transplant recipients is associated with progressive graft dysfunction and graft loss. Cidofovir, an antiviral agent with known nephrotoxicity, has been used in low doses to treat BKV infections. However, the systemic exposure and disposition of the low-dose cidofovir regimen are not known in kidney transplant recipients.
Methods: We investigated the pharmacokinetics (PK) of low-dose cidofovir (0.24 - 0.62 mg/kg) both without and with oral probenecid in 9 transplant patients with persistent BK viremia without nephropathy in a crossover design.
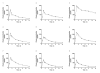
Results: The mean estimated glomerular filtration rate (eGFR) of the study participants was 46.2 mL/min/1.73 m(2) (range: 17-75 mL/min/1.73 m(2) ). The contribution of active renal secretion to cidofovir total body clearance was assessed by evaluating the effect of probenecid on cidofovir PK. Maximum cidofovir plasma concentrations, which averaged approximately 1 μg/mL, were significantly below the 36 μg/mL 50% effective concentration in vitro for cidofovir against BKV. The plasma concentration of cidofovir declined with an overall disposition half-life of 5.1 ± 3.5 and 5.3 ± 2.9 h in the absence and in the presence of probenecid, respectively (P > 0.05).
Conclusions: Cidofovir clearance and eGFR were linearly related irrespective of probenecid administration (r(2) = 0.8 without probenecid; r(2) = 0.7 with probenecid). This relationship allows for the prediction of systemic cidofovir exposure in individual patients and may be utilized to evaluate exposure-response relationships to optimize the cidofovir dosing regimen for BKV infection.
© 2012 John Wiley & Sons A/S.
Figures
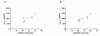
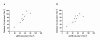
References
-
- Nickeleit V, Hirsch HH, Zeiler M, et al. BK-virus nephropathy in renal transplantstubular necrosis, MHC-class II expression and rejection in a puzzling game. Nephrol Dial Transplant. 2000;15(3):324–332. - PubMed
-
- Purighalla R, Shapiro R, McCauley J, Randhawa P. BK virus infection in a kidney allograft diagnosed by needle biopsy. Am J Kidney Dis. 1995;26(4):671–673. - PubMed
-
- Ramos E, Drachenberg CB, Papadimitriou JC, et al. Clinical course of polyoma virus nephropathy in 67 renal transplant patients. J Am Soc Nephrol. 2002;13(8):2145–2151. - PubMed
-
- Randhawa PS, Finkelstein S, Scantlebury V, et al. Human polyoma virus-associated interstitial nephritis in the allograft kidney. Transplantation. 1999;67(1):103–109. - PubMed
-
- Randhawa P, Brennan DC. BK virus infection in transplant recipients: an overview and update. Am J Transplant. 2006;6(9):2000–2005. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

