Blast wave exposure impairs memory and decreases axon initial segment length
- PMID: 23025758
- PMCID: PMC3941920
- DOI: 10.1089/neu.2012.2478
Blast wave exposure impairs memory and decreases axon initial segment length
Abstract
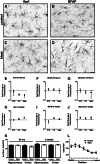
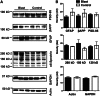
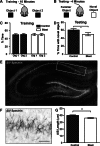
Exposure to a blast wave has been proposed to cause mild traumatic brain injury (mTBI), with symptoms including altered cognition, memory, and behavior. This idea, however, remains controversial, and the mechanisms of blast-induced brain injury remain unknown. To begin to resolve these questions, we constructed a simple compressed air shock tube, placed rats inside the tube, and exposed them to a highly reproducible and controlled blast wave. Consistent with the generation of a mild injury, 2 weeks after exposure to the blast, we found that motor performance was unaffected, and a panel of common injury markers showed little or no significant changes in expression in the cortex, corpus callosum, or hippocampus. Similarly, we were unable to detect elevated spectrin breakdown products in brains collected from blast-exposed rats. Using an object recognition task, however, we found that rats exposed to a blast wave spent significantly less time exploring a novel object when compared with control rats. Intriguingly, we also observed a significant shortening of the axon initial segment (AIS) in both the cortex and hippocampus of blast-exposed rats, suggesting altered neuronal excitability after exposure to a blast. A computational model showed that shortening the AIS increased both threshold and the interspike interval of repetitively firing neurons. These results support the conclusion that exposure to a single blast wave can lead to mTBI with accompanying cognitive impairment and subcellular changes in the molecular organization of neurons.
Figures




References
-
- Okie S. Traumatic brain injury in the war zone. N. Engl. J. Med. 2005;352:2043–2047. - PubMed
-
- Ling G.S. Ecklund J.M. Traumatic brain injury in modern war. Curr. Opin. Anaesthesiol. 2011;24:124–130. - PubMed
-
- Elsayed N.M. Toxicology of blast overpressure. Toxicology. 1997;121:1–15. - PubMed
-
- Mayorga M.A. The pathology of primary blast overpressure injury. Toxicology. 1997;121:17–28. - PubMed
-
- Leung L.Y. VandeVord P.J. Dal Cengio A.L. Bir C. Yang K.H. King A.I. Blast related neurotrauma: a review of cellular injury. Mol Cell Biomech. 2008;5:155–168. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

