Chromosome anomalies in bone marrow as primary cause of aplastic or hypoplastic conditions and peripheral cytopenia: disorders due to secondary impairment of RUNX1 and MPL genes
- PMID: 23025896
- PMCID: PMC3542585
- DOI: 10.1186/1755-8166-5-39
Chromosome anomalies in bone marrow as primary cause of aplastic or hypoplastic conditions and peripheral cytopenia: disorders due to secondary impairment of RUNX1 and MPL genes
Abstract
Background: Chromosome changes in the bone marrow (BM) of patients with persistent cytopenia are often considered diagnostic for a myelodysplastic syndrome (MDS). Comprehensive cytogenetic evaluations may give evidence of the real pathogenetic role of these changes in cases with cytopenia without morphological signs of MDS.
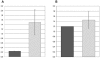
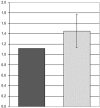
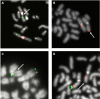
Results: Chromosome anomalies were found in the BM of three patients, without any morphological evidence of MDS: 1) an acquired complex rearrangement of chromosome 21 in a boy with severe aplastic anaemia (SAA); the rearrangement caused the loss of exons 2-8 of the RUNX1 gene with subsequent hypoexpression. 2) a constitutional complex rearrangement of chromosome 21 in a girl with congenital thrombocytopenia; the rearrangement led to RUNX1 disruption and hypoexpression. 3) an acquired paracentric inversion of chromosome 1, in which two regions at the breakpoints were shown to be lost, in a boy with aplastic anaemia; the MPL gene, localized in chromosome 1 short arms was not mutated neither disrupted, but its expression was severely reduced: we postulate that the aplastic anaemia was due to position effects acting both in cis and in trans, and causing Congenital Amegakaryocytic Thrombocytopenia (CAMT).
Conclusions: A clonal anomaly in BM does not imply per se a diagnosis of MDS: a subgroup of BM hypoplastic disorders is directly due to chromosome structural anomalies with effects on specific genes, as was the case of RUNX1 and MPL in the patients here reported with diagnosis of SAA, thrombocytopenia, and CAMT. The anomaly may be either acquired or constitutional, and it may act by deletion/disruption of the gene, or by position effects. Full cytogenetic investigations, including a-CGH, should always be part of the diagnostic evaluation of patients with BM aplasia/hypoplasia and peripheral cytopenias.
Figures
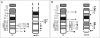


References
-
- Vardiman JW, Thiele J, Arber DA, Brunning RD, Borowitz MJ, Porwit A, Harris NL, Le Beau MM, Hellström-Lindberg E, Tefferi A, Bloomfield CD. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;30:937–951. - PubMed
-
- Maserati E, Panarello C, Morerio C, Valli R, Pressato B, Patitucci F, Tassano E, Di Cesare-Merlone A, Cugno C, Balduini CL, Lo Curto F, Dufour C, Locatelli F, Pasquali F. Clonal chromosome anomalies and propensity to myeloid malignancies in Congenital Amegakaryocytic Thrombocytopenia (OMIM # 604498) Haematologica. 2008;93:1271–1273. doi: 10.3324/haematol.12748. - DOI - PubMed
-
- Noris P, Perrotta S, Seri M, Pecci A, Gnan C, Loffredo G, Pujol-Moix N, Zecca M, Scognamiglio F, De Rocco D, Punzo F, Melazzini F, Scianguetta S, Casale M, Marconi C, Pippucci T, Amendola G, Notarangelo LD, Klersy C, Civaschi E, Balduini CL, Savoia A. Mutations in ANKRD26 are responsible for a frequent form of inherited thrombocytopenia: analysis of 78 patients from 21 families. Blood. 2011;117:6673–6680. doi: 10.1182/blood-2011-02-336537. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

