Emerging roles of Nrf2 and phase II antioxidant enzymes in neuroprotection
- PMID: 23025925
- PMCID: PMC3623606
- DOI: 10.1016/j.pneurobio.2012.09.003
Emerging roles of Nrf2 and phase II antioxidant enzymes in neuroprotection
Abstract
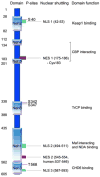
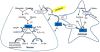
Phase II metabolic enzymes are a battery of critical proteins that detoxify xenobiotics by increasing their hydrophilicity and enhancing their disposal. These enzymes have long been studied for their preventative and protective effects against mutagens and carcinogens and for their regulation via the Keap1 (Kelch-like ECH associated protein 1)/Nrf2 (Nuclear factor erythroid 2 related factor 2)/ARE (antioxidant response elements) pathway. Recently, a series of studies have reported the altered expression of phase II genes in postmortem tissue of patients with various neurological diseases. These observations hint at a role for phase II enzymes in the evolution of such conditions. Furthermore, promising findings reveal that overexpression of phase II genes, either by genetic or chemical approaches, confers neuroprotection in vitro and in vivo. Therefore, there is a need to summarize the current literature on phase II genes in the central nervous system (CNS). This should help guide future studies on phase II genes as therapeutic targets in neurological diseases. In this review, we first briefly introduce the concept of phase I, II and III enzymes, with a special focus on phase II enzymes. We then discuss their expression regulation, their inducers and executors. Following this background, we expand our discussion to the neuroprotective effects of phase II enzymes and the potential application of Nrf2 inducers to the treatment of neurological diseases.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures



References
-
- Ahlgren-Beckendorf JA, Reising AM, Schander MA, Herdler JW, Johnson JA. Coordinate regulation of NAD(P)H:quinone oxidoreductase and glutathione-S-transferases in primary cultures of rat neurons and glia: role of the antioxidant/electrophile responsive element. Glia. 1999;25:131–142. - PubMed
-
- Alam J, Wicks C, Stewart D, Gong P, Touchard C, Otterbein S, Choi AM, Burow ME, Tou J. Mechanism of heme oxygenase-1 gene activation by cadmium in MCF-7 mammary epithelial cells. Role of p38 kinase and Nrf2 transcription factor. J Biol Chem. 2000;275:27694–27702. - PubMed
-
- Anderson MF, Blomstrand F, Blomstrand C, Eriksson PS, Nilsson M. Astrocytes and stroke: networking for survival? Neurochem Res. 2003;28:293–305. - PubMed
-
- Andoh T, Chock PB, Chiueh CC. The roles of thioredoxin in protection against oxidative stress-induced apoptosis in SH-SY5Y cells. J Biol Chem. 2002;277:9655–9660. - PubMed
-
- Aoyama K, Watabe M, Nakaki T. Regulation of neuronal glutathione synthesis. J Pharmacol Sci. 2008;108:227–238. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

