Delving deeper: MCL-1's contributions to normal and cancer biology
- PMID: 23026029
- PMCID: PMC3532576
- DOI: 10.1016/j.tcb.2012.08.011
Delving deeper: MCL-1's contributions to normal and cancer biology
Abstract
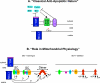
BCL-2 molecules are regulators of programmed cell death and defects in this pathway contribute to human diseases. One family member, MCL-1, is unique because its expression is tightly regulated and it is essential for promoting the survival of myriad cellular lineages. Additionally, MCL-1 promotes the maintenance of normal mitochondrial morphology and energy production. Dissection of these functions revealed recently that they depend on separate mitochondrial sublocalizations. MCL-1's antiapoptotic activity is restricted to the outer mitochondrial membrane (OMM), whereas its function in mitochondrial physiology requires localization to the matrix. These findings provide an attractive model for how MCL-1's diverse functions may contribute to normal cell homeostasis and function. MCL-1 is highly amplified in human cancer, suggesting that these functions may contribute to malignant cell growth and evasion of apoptosis.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures


References
-
- Cory S, Adams JM. The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer. 2002;2:647–656. - PubMed
-
- Cheng EH, et al. BCL-2, BCL-X(L) sequester BH3 domain-only molecules preventing BAX- and BAK-mediated mitochondrial apoptosis. Mol Cell. 2001;8:705–711. - PubMed
-
- Goldstein JC, et al. The coordinate release of cytochrome c during apoptosis is rapid, complete and kinetically invariant. Nat Cell Biol. 2000;2:156–162. - PubMed
-
- Day CL, et al. Structure of the BH3 domains from the p53-inducible BH3-only proteins Noxa and Puma in complex with Mcl-1. J Mol Biol. 2008;380:958–971. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

