Ultrastructure of human mature oocytes after vitrification
- PMID: 23027354
- PMCID: PMC3493984
- DOI: 10.4081/ejh.2012.e38
Ultrastructure of human mature oocytes after vitrification
Abstract
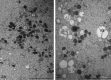
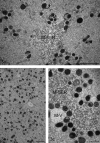
Since the introduction of human assisted reproduction, oocyte cryopreservation has been regarded as an attractive option to capitalize the reproductive potential of surplus oocytes and preserve female fertility. However, for two decades the endeavor to store oocytes has been limited by the not yet optimized methodologies, with the consequence of poor clinical outcome or of uncertain reproducibility. Vitrification has been developed as the promising technology of cryopreservation even if slow freezing remains a suitable choice. Nevertheless, the insufficiency of clinical and correlated multidisciplinary data is still stirring controversy on the impact of this technique on oocyte integrity. Morphological studies may actually provide a great insight in this debate. Phase contrast microscopy and other light microscopy techniques, including cytochemistry, provided substantial morphofunctional data on cryopreserved oocyte, but are unable to unraveling fine structural changes. The ultrastructural damage is one of the most adverse events associated with cryopreservation, as an effect of cryo-protectant toxicity, ice crystal formation and osmotic stress. Surprisingly, transmission electron microscopy has attracted only limited attention in the field of cryopreservation. In this review, the subcellular structure of human mature oocytes following vitrification is discussed at the light of most relevant ultrastructural studies.
Conflict of interest statement
Conflict of interests: all the authors declare there is no conflict of interest, personal, financial or otherwise, in relation of this work.
Figures


References
-
- Smith GD, Motta EE, Serafini P. Theoretical and experimental basis of oocyte vitrification. Reprod Biomed Online. 2011;23:298–306. - PubMed
-
- Gosden R. Cryopreservation: a cold look at technology for fertility preservation. Fertil Steril. 2011;96:264–8. - PubMed
-
- Cobo A, Diaz C. Clinical application of oocyte vitrification: a systematic review and meta-analysis of randomized controlled trials. Fertil Steril. 2011;96:277–85. - PubMed
-
- Herrero L, Martínez M, Garcia-Velasco JA. Current status of human oocyte and embryo cryopreservation. Curr Opin Obstet Gynecol. 2011;23:245–50. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

