Microbial amyloids induce interleukin 17A (IL-17A) and IL-22 responses via Toll-like receptor 2 activation in the intestinal mucosa
- PMID: 23027540
- PMCID: PMC3497426
- DOI: 10.1128/IAI.00911-12
Microbial amyloids induce interleukin 17A (IL-17A) and IL-22 responses via Toll-like receptor 2 activation in the intestinal mucosa
Abstract
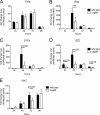
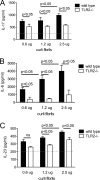
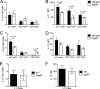
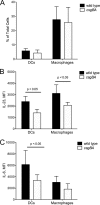
The Toll-like receptor 2 (TLR2)/TLR1 receptor complex responds to amyloid fibrils, a common component of biofilm material produced by members of the phyla Firmicutes, Bacteroidetes, and Proteobacteria. To determine whether this TLR2/TLR1 ligand stimulates inflammatory responses when bacteria enter intestinal tissue, we investigated whether expression of curli amyloid fibrils by the invasive enteric pathogen Salmonella enterica serotype Typhimurium contributes to T helper 1 and T helper 17 responses by measuring cytokine production in the mouse colitis model. A csgBA mutant, deficient in curli production, elicited decreased expression of interleukin 17A (IL-17A) and IL-22 in the cecal mucosa compared to the S. Typhimurium wild type. In TLR2-deficient mice, IL-17A and IL-22 expression was blunted during S. Typhimurium infection, suggesting that activation of the TLR2 signaling pathway contributes to the expression of these cytokines. T cells incubated with supernatants from bone marrow-derived dendritic cells (BMDCs) treated with curli fibrils released IL-17A in a TLR2-dependent manner in vitro. Lower levels of IL-6 and IL-23 production were detected in the supernatants of the TLR2-deficient BMDCs treated with curli fibrils. Consistent with this, three distinct T-cell populations-CD4(+) T helper cells, cytotoxic CD8(+) T cells, and γδ T cells-produced IL-17A in response to curli fibrils in the intestinal mucosa during S. Typhimurium infection. Notably, decreased IL-6 expression by the dendritic cells and decreased IL-23 expression by the dendritic cells and macrophages were observed in the cecal mucosa of mice infected with the curli mutant. We conclude that TLR2 recognition of bacterial amyloid fibrils in the intestinal mucosa represents a novel mechanism of immunoregulation, which contributes to the generation of inflammatory responses, including production of IL-17A and IL-22, in response to bacterial entry into the intestinal mucosa.
Figures



References
-
- Aliprantis AO, et al. 1999. Cell activation and apoptosis by bacterial lipoproteins through Toll-like receptor-2. Science 285:736–739 - PubMed
-
- Bettelli E, et al. 2006. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441:235–238 - PubMed
-
- Bian Z, Brauner A, Li Y, Normark S. 2000. Expression of and cytokine activation by Escherichia coli curli fibers in human sepsis. J. Infect. Dis. 181:602–612 - PubMed
-
- Bian Z, Yan ZQ, Hansson GK, Thoren P, Normark S. 2001. Activation of inducible nitric oxide synthase/nitric oxide by curli fibers leads to a fall in blood pressure during systemic Escherichia coli infection in mice. J. Infect. Dis. 183:612–619 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI050553/AI/NIAID NIH HHS/United States
- AI082320/AI/NIAID NIH HHS/United States
- R56 AI047325/AI/NIAID NIH HHS/United States
- R01 AI052306/AI/NIAID NIH HHS/United States
- R21 AI082320/AI/NIAID NIH HHS/United States
- U54 AI57168/AI/NIAID NIH HHS/United States
- R01 AI084065/AI/NIAID NIH HHS/United States
- AI047325/AI/NIAID NIH HHS/United States
- AI052306/AI/NIAID NIH HHS/United States
- AI084065/AI/NIAID NIH HHS/United States
- R01 AI047325/AI/NIAID NIH HHS/United States
- U54 AI057168/AI/NIAID NIH HHS/United States
- AI050553/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

