Localization of gonadotropin-releasing hormone (GnRH), gonadotropin-inhibitory hormone (GnIH), kisspeptin and GnRH receptor and their possible roles in testicular activities from birth to senescence in mice
- PMID: 23027641
- PMCID: PMC3640405
- DOI: 10.1002/jez.1765
Localization of gonadotropin-releasing hormone (GnRH), gonadotropin-inhibitory hormone (GnIH), kisspeptin and GnRH receptor and their possible roles in testicular activities from birth to senescence in mice
Abstract
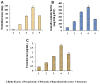
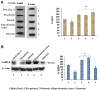
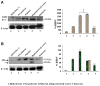
The changes in distribution and concentration of neuropeptides, gonadotropin-releasing hormone (GnRH), gonadotropin-inhibitory hormone (GnIH), kisspeptin, and gonadotropin-releasing hormone receptor (GnRH-R) were evaluated and compared with reproductive parameters, such as cytochrome P450 side-chain cleavage (P450 SCC) enzyme activity, androgen receptors (AR) in the testis and serum testosterone levels, from birth to senescence in mice. The results showed the localization of these molecules mainly in the interstitial and germ cells as well as showed significant variations in immunostatining from birth to senescence. It was found that increased staining of testicular GnRH-R coincided with increased steroidogenic activity during pubertal and adult stages, whereas decreased staining coincides with decreased steroidogenic activity during senescence. Similar changes in immunostaining were confirmed by Western/slot blot analysis. Thus, these results suggest a putative role of GnRH during testicular pubertal development and senescence. Treatment with a GnRH agonist ([DTrp(6), Pro(9)-NEt] GnRH) to mice from prepubertal to pubertal period showed a significant increase in steroidogenic activity of the mouse testis and provided further support to the role of GnRH in testicular pubertal maturation. The significant decline in GnRH-R during senescence may be due to a significant increase in GnIH synthesis during senescence causing the decrease in GnRH-R expression. It is considered that significant changes in the levels of GnRH-R may be responsible for changes in steroidogenesis that causes either pubertal activation or senescence in testis of mice. Furthermore, changes in the levels of GnRH-R may be modulated by interactions among GnRH, GnIH, and kisspeptin in the testis.
Copyright © 2012 Wiley Periodicals, Inc.
Figures




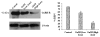
References
-
- Adams TE. Using gonadotropin-releasing hormone (GnRH) and GnRH analogs to modulate testis function and enhance the productivity of domestic animals. Anim Reprod Sci. 2005;88:127–139. - PubMed
-
- Banerjee A, Singh A, Srivastava P, Turner H, Krishna A. Effects of chronic bhang (cannabis) administration on the reproductive system of male mice. Birth Defect Res B. 2011;92:195–205. - PubMed
-
- Bélanger A, Candas B, Dupont A, et al. Changes in serum concentrations of conjugated and unconjugated steroids in 40- to 80-year-old men. J Clin Endocrinol Metab. 1994;79:1086–1090. - PubMed
-
- Bhasin S, Heber D, Peterson M, Swerdloff R. Partial isolation and characterization of testicular GnRH-like factors. Endocrinology. 1983;112:1144–1146. - PubMed
-
- Botte MC, Chamagne AM, Carre MC, Counis R, Kottler ML. Fetal expression of GnRH and GnRH receptor genes in rat testis and ovary. J Endocrinol. 1998;159:179–189. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

