UDP-glycosyltransferases from the UGT73C subfamily in Barbarea vulgaris catalyze sapogenin 3-O-glucosylation in saponin-mediated insect resistance
- PMID: 23027665
- PMCID: PMC3510118
- DOI: 10.1104/pp.112.202747
UDP-glycosyltransferases from the UGT73C subfamily in Barbarea vulgaris catalyze sapogenin 3-O-glucosylation in saponin-mediated insect resistance
Abstract
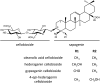
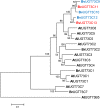
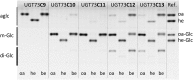
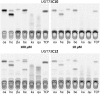
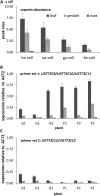
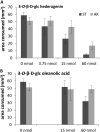
Triterpenoid saponins are bioactive metabolites that have evolved recurrently in plants, presumably for defense. Their biosynthesis is poorly understood, as is the relationship between bioactivity and structure. Barbarea vulgaris is the only crucifer known to produce saponins. Hederagenin and oleanolic acid cellobioside make some B. vulgaris plants resistant to important insect pests, while other, susceptible plants produce different saponins. Resistance could be caused by glucosylation of the sapogenins. We identified four family 1 glycosyltransferases (UGTs) that catalyze 3-O-glucosylation of the sapogenins oleanolic acid and hederagenin. Among these, UGT73C10 and UGT73C11 show highest activity, substrate specificity and regiospecificity, and are under positive selection, while UGT73C12 and UGT73C13 show lower substrate specificity and regiospecificity and are under purifying selection. The expression of UGT73C10 and UGT73C11 in different B. vulgaris organs correlates with saponin abundance. Monoglucosylated hederagenin and oleanolic acid were produced in vitro and tested for effects on P. nemorum. 3-O-β-d-Glc hederagenin strongly deterred feeding, while 3-O-β-d-Glc oleanolic acid only had a minor effect, showing that hydroxylation of C23 is important for resistance to this herbivore. The closest homolog in Arabidopsis thaliana, UGT73C5, only showed weak activity toward sapogenins. This indicates that UGT73C10 and UGT73C11 have neofunctionalized to specifically glucosylate sapogenins at the C3 position and demonstrates that C3 monoglucosylation activates resistance. As the UGTs from both the resistant and susceptible types of B. vulgaris glucosylate sapogenins and are not located in the known quantitative trait loci for resistance, the difference between the susceptible and resistant plant types is determined at an earlier stage in saponin biosynthesis.
Figures
References
-
- Agerbirk N, Olsen CE, Bibby BM, Frandsen HO, Brown LD, Nielsen JK, Renwick JAA. (2003a) A saponin correlated with variable resistance of Barbarea vulgaris to the diamondback moth Plutella xylostella. J Chem Ecol 29: 1417–1433 - PubMed
-
- Agerbirk N, Ørgaard M, Nielsen JK. (2003b) Glucosinolates, P. nemorum resistance, and leaf pubescence as taxonomic characters in the genus Barbarea (Brassicaceae). Phytochemistry 63: 69–80 - PubMed
-
- Aharoni A, Gaidukov L, Khersonsky O, McQ Gould S, Roodveldt C, Tawfik DS. (2005) The ‘evolvability’ of promiscuous protein functions. Nat Genet. 371: 73–76 - PubMed
-
- Augustin JM, Kuzina V, Andersen SB, Bak S. (2011) Molecular activities, biosynthesis and evolution of triterpenoid saponins. Phytochemistry 72: 435–457 - PubMed
-
- Badenes-Perez FR, Shelton AM, Nault BA. (2005) Using yellow rocket as a trap crop for diamondback moth (Lepidoptera: Plutellidae). J Econ Entomol 98: 884–890 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

