Quantifying Ca2+ release and inactivation of Ca2+ release in fast- and slow-twitch muscles
- PMID: 23027818
- PMCID: PMC3530126
- DOI: 10.1113/jphysiol.2012.242073
Quantifying Ca2+ release and inactivation of Ca2+ release in fast- and slow-twitch muscles
Abstract
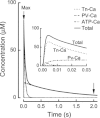
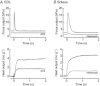
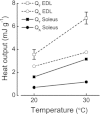
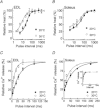
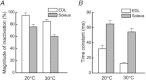
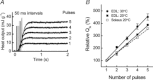
The aims of this study were to quantify the Ca(2+) release underlying twitch contractions of mammalian fast- and slow-twitch muscle and to comprehensively describe the transient inactivation of Ca(2+) release following a stimulus. Experiments were performed using bundles of fibres from mouse extensor digitorum longus (EDL) and soleus muscles. Ca(2+) release was quantified from the amount of ATP used to remove Ca(2+) from the myoplasm following stimulation. ATP turnover by crossbridges was blocked pharmacologically (N-benzyl-p-toluenesulphonamide for EDL, blebbistatin for soleus) and muscle heat production was used as an index of Ca(2+) pump ATP turnover. At 20°C, Ca(2+) release in response to a single stimulus was 34 and 84 μmol (kg muscle)(-1) for soleus and EDL, respectively, and increased with temperature (30°C: soleus, 61 μmol kg(-1); EDL, 168 μmol kg(-1)). Delivery of another stimulus within 100 ms of the first produced a smaller Ca(2+) release. The maximum magnitude of the decrease in Ca(2+) release was greater in EDL than soleus. Ca(2+) release recovered with an exponential time course which was faster in EDL (mean time constant at 20°C, 32.1 ms) than soleus (65.6 ms) and faster at 30°C than at 20°C. The amounts of Ca(2+) released and crossbridge cycles performed are consistent with a scheme in which Ca(2+) binding to troponin-C allowed an average of ∼1.7 crossbridge cycles in the two muscles.
Figures
References
-
- Barclay CJ. Modelling diffusive O2 supply to isolated preparations of mammalian skeletal and cardiac muscle. J Muscle Res Cell Motil. 2005;26:225–235. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

