Interplay between menin and K-Ras in regulating lung adenocarcinoma
- PMID: 23027861
- PMCID: PMC3501031
- DOI: 10.1074/jbc.M112.382416
Interplay between menin and K-Ras in regulating lung adenocarcinoma
Abstract
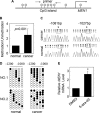
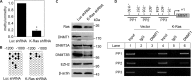
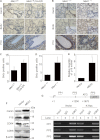
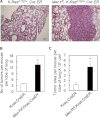
MEN1, which encodes the nuclear protein menin, acts as a tumor suppressor in lung cancer and is often inactivated in human primary lung adenocarcinoma. Here, we show that the inactivation of MEN1 is associated with increased DNA methylation at the MEN1 promoter by K-Ras. On one hand, the activated K-Ras up-regulates the expression of DNA methyltransferases and enhances the binding of DNA methyltransferase 1 to the MEN1 promoter, leading to increased DNA methylation at the MEN1 gene in lung cancer cells; on the other hand, menin reduces the level of active Ras-GTP at least partly by preventing GRB2 and SOS1 from binding to Ras, without affecting the expression of GRB2 and SOS1. In human lung adenocarcinoma samples, we further demonstrate that reduced menin expression is associated with the enhanced expression of Ras (p < 0.05). Finally, excision of the Men1 gene markedly accelerates the K-Ras(G12D)-induced tumor formation in the Men1(f/f);K-Ras(G12D/+);Cre ER mouse model. Together, these findings uncover a previously unknown link between activated K-Ras and menin, an important interplay governing tumor activation and suppression in the development of lung cancer.
Figures


References
-
- Jemal A., Bray F., Center M. M., Ferlay J., Ward E., Forman D. (2011) Global Cancer Statistics. CA-Cancer J. Clin. 61, 69–90 - PubMed
-
- Jemal A., Thomas A., Murray T., Thun M. (2002) Cancer statistics, 2002. CA-Cancer J. Clin. 52, 23–47 - PubMed
-
- Soda M., Choi Y. L., Enomoto M., Takada S., Yamashita Y., Ishikawa S., Fujiwara S., Watanabe H., Kurashina K., Hatanaka H., Bando M., Ohno S., Ishikawa Y., Aburatani H., Niki T., Sohara Y., Sugiyama Y., Mano H. (2007) Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature 448, 561–566 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

