Cross-talk among RNA polymerase II kinases modulates C-terminal domain phosphorylation
- PMID: 23027873
- PMCID: PMC3493918
- DOI: 10.1074/jbc.M112.412015
Cross-talk among RNA polymerase II kinases modulates C-terminal domain phosphorylation
Abstract
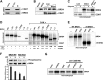
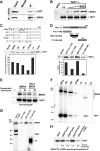
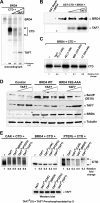
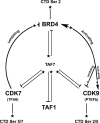
The RNA polymerase II (Pol II) C-terminal domain (CTD) serves as a docking site for numerous proteins, bridging various nuclear processes to transcription. The recruitment of these proteins is mediated by CTD phospho-epitopes generated during transcription. The mechanisms regulating the kinases that establish these phosphorylation patterns on the CTD are not known. We report that three CTD kinases, CDK7, CDK9, and BRD4, engage in cross-talk, modulating their subsequent CTD phosphorylation. BRD4 phosphorylates PTEFb/CDK9 at either Thr-29 or Thr-186, depending on its relative abundance, which represses or activates CDK9 CTD kinase activity, respectively. Conversely, CDK9 phosphorylates BRD4 enhancing its CTD kinase activity. The CTD Ser-5 kinase CDK7 also interacts with and phosphorylates BRD4, potently inhibiting BRD4 kinase activity. Additionally, the three kinases regulate each other indirectly through the general transcription factor TAF7. An inhibitor of CDK9 and CDK7 CTD kinase activities, TAF7 also binds to BRD4 and inhibits its kinase activity. Each of these kinases phosphorylates TAF7, affecting its subsequent ability to inhibit the other two. Thus, a complex regulatory network governs Pol II CTD kinases.
Figures


 ), arrowheads (
), arrowheads ( ), and cross-bars (⊣), respectively.
), and cross-bars (⊣), respectively.References
-
- Phatnani H. P., Greenleaf A. L. (2006) Phosphorylation and functions of the RNA polymerase II CTD. Genes Dev. 20, 2922–2936 - PubMed
-
- Bataille A. R., Jeronimo C., Jacques P. É., Laramée L., Fortin M. È., Forest A., Bergeron M., Hanes S. D., Robert F. (2012) A universal RNA polymerase II CTD cycle is orchestrated by complex interplays between kinase, phosphatase, and isomerase enzymes along genes. Mol. Cell 45, 158–170 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

