Neuroprotective efficacy of aminopropyl carbazoles in a mouse model of Parkinson disease
- PMID: 23027934
- PMCID: PMC3479520
- DOI: 10.1073/pnas.1213956109
Neuroprotective efficacy of aminopropyl carbazoles in a mouse model of Parkinson disease
Abstract
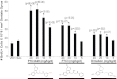
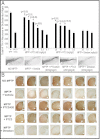
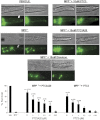
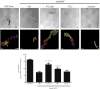
We previously reported the discovery of P7C3, an aminopropyl carbazole having proneurogenic and neuroprotective properties in newborn neural precursor cells of the dentate gyrus. Here, we provide evidence that P7C3 also protects mature neurons in brain regions outside of the hippocampus. P7C3 blocks 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-mediated cell death of dopaminergic neurons in the substantia nigra of adult mice, a model of Parkinson disease (PD). Dose-response studies show that the P7C3 analog P7C3A20 blocks cell death with even greater potency and efficacy, which parallels the relative potency and efficacy of these agents in blocking apoptosis of newborn neural precursor cells of the dentate gyrus. P7C3 and P7C3A20 display similar relative effects in blocking 1-methyl-4-phenylpyridinium (MPP(+))-mediated death of dopaminergic neurons in Caenorhabditis elegans, as well as in preserving C. elegans mobility following MPP(+) exposure. Dimebon, an antihistaminergic drug that is weakly proneurogenic and neuroprotective in the dentate gyrus, confers no protection in either the mouse or the worm models of PD. We further demonstrate that the hippocampal proneurogenic efficacy of eight additional analogs of P7C3 correlates with their protective efficacy in MPTP-mediated neurotoxicity. In vivo screening of P7C3 analogs for proneurogenic efficacy in the hippocampus may thus provide a reliable means of predicting neuroprotective efficacy. We propose that the chemical scaffold represented by P7C3 and P7C3A20 provides a basis for optimizing and advancing pharmacologic agents for the treatment of patients with PD.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Comment in
-
Neurodegenerative diseases: Novel route to neuroprotection.Nat Rev Drug Discov. 2012 Dec;11(12):906-7. doi: 10.1038/nrd3899. Nat Rev Drug Discov. 2012. PMID: 23197030 No abstract available.
References
-
- Lees AJ, Hardy J, Revesz T. Parkinson’s disease. Lancet. 2009;373:2055–2066. - PubMed
-
- McKnight SL, Pieper AA, Ready JM, De Brabander J. 2010. Proneurogenic compounds. US Patent 2010/020681.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

