Analysis of human samples reveals impaired SHH-dependent cerebellar development in Joubert syndrome/Meckel syndrome
- PMID: 23027964
- PMCID: PMC3479472
- DOI: 10.1073/pnas.1201408109
Analysis of human samples reveals impaired SHH-dependent cerebellar development in Joubert syndrome/Meckel syndrome
Abstract
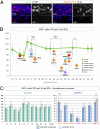
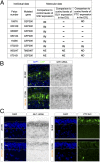
Joubert syndrome (JS) and Meckel syndrome (MKS) are pleiotropic ciliopathies characterized by severe defects of the cerebellar vermis, ranging from hypoplasia to aplasia. Interestingly, ciliary conditional mutant mice have a hypoplastic cerebellum in which the proliferation of cerebellar granule cell progenitors (GCPs) in response to Sonic hedgehog (SHH) is severely reduced. This suggests that Shh signaling defects could contribute to the vermis hypoplasia observed in the human syndromes. As existing JS/MKS mutant mouse models suggest apparently contradictory hypotheses on JS/MKS etiology, we investigated Shh signaling directly on human fetal samples. First, in an examination of human cerebellar development, we linked the rates of GCP proliferation to the different levels and localizations of active Shh signaling and showed that the GCP possessed a primary cilium with CEP290 at its base. Second, we found that the proliferation of GCPs and their response to SHH were severely impaired in the cerebellum of subjects with JS/MKS and Jeune syndrome. Finally, we showed that the defect in GCP proliferation was similar in the cerebellar vermis and hemispheres in all patients with ciliopathy analyzed, suggesting that the specific cause of vermal hypo-/aplasia precedes this defect. Our results, obtained from the analysis of human samples, show that the hemispheres and the vermis are affected in JS/MKS and provide evidence of a defective cellular mechanism in these pathologic processes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Louie CM, Gleeson JG. Genetic basis of Joubert syndrome and related disorders of cerebellar development. Hum Mol Genet. 2005;14 spec no 2:R235–R242. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Research Materials

