Bacillus cereus iron uptake protein fishes out an unstable ferric citrate trimer
- PMID: 23027976
- PMCID: PMC3479497
- DOI: 10.1073/pnas.1210131109
Bacillus cereus iron uptake protein fishes out an unstable ferric citrate trimer
Abstract
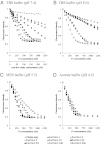
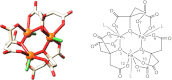
Citrate is a common biomolecule that chelates Fe(III). Many bacteria and plants use ferric citrate to fulfill their nutritional requirement for iron. Only the Escherichia coli ferric citrate outer-membrane transport protein FecA has been characterized; little is known about other ferric citrate-binding proteins. Here we report a unique siderophore-binding protein from the gram-positive pathogenic bacterium Bacillus cereus that binds multinuclear ferric citrate complexes. We have demonstrated that B. cereus ATCC 14579 takes up (55)Fe radiolabeled ferric citrate and that a protein, BC_3466 [renamed FctC (ferric citrate-binding protein C)], binds ferric citrate. The dissociation constant (K(d)) of FctC at pH 7.4 with ferric citrate (molar ratio 1:50) is 2.6 nM. This is the tightest binding observed of any B. cereus siderophore-binding protein. Nano electrospray ionization-mass spectrometry (nano ESI-MS) analysis of FctC and ferric citrate complexes or citrate alone show that FctC binds diferric di-citrate, and triferric tricitrate, but does not bind ferric di-citrate, ferric monocitrate, or citrate alone. Significantly, the protein selectively binds triferric tricitrate even though this species is naturally present at very low equilibrium concentrations.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Raymond KN, Dertz EA. Biochemical and physical properties of siderophores. In: Crosa JH, Mey AR, Payne SM, editors. Iron Transport in Bacteria. Washington, DC: ASM Press; 2004. pp. 3–17.
-
- Braun V, Hantke K, Koster W. Bacterial iron transport: Mechanisms, genetics, and regulation. In: Sigel A, Sigel H, editors. Metal Ions in Biological Systems. Vol 35. New York: Marcel Dekker, Inc.; 1998. pp. 67–145. - PubMed
-
- Koppisch AT, et al. Petrobactin is the primary siderophore synthesized by Bacillus anthracis str. Sterne under conditions of iron starvation. Biometals. 2005;18:577–585. - PubMed
-
- Müller SI, Valdebenito M, Hantke K. Salmochelin, the long-overlooked catecholate siderophore of Salmonella. Biometals. 2009;22:691–695. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

