Correlating microbial community profiles with geochemical data in highly stratified sediments from the Arctic Mid-Ocean Ridge
- PMID: 23027979
- PMCID: PMC3479504
- DOI: 10.1073/pnas.1207574109
Correlating microbial community profiles with geochemical data in highly stratified sediments from the Arctic Mid-Ocean Ridge
Abstract
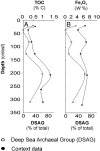
Microbial communities and their associated metabolic activity in marine sediments have a profound impact on global biogeochemical cycles. Their composition and structure are attributed to geochemical and physical factors, but finding direct correlations has remained a challenge. Here we show a significant statistical relationship between variation in geochemical composition and prokaryotic community structure within deep-sea sediments. We obtained comprehensive geochemical data from two gravity cores near the hydrothermal vent field Loki's Castle at the Arctic Mid-Ocean Ridge, in the Norwegian-Greenland Sea. Geochemical properties in the rift valley sediments exhibited strong centimeter-scale stratigraphic variability. Microbial populations were profiled by pyrosequencing from 15 sediment horizons (59,364 16S rRNA gene tags), quantitatively assessed by qPCR, and phylogenetically analyzed. Although the same taxa were generally present in all samples, their relative abundances varied substantially among horizons and fluctuated between Bacteria- and Archaea-dominated communities. By independently summarizing covariance structures of the relative abundance data and geochemical data, using principal components analysis, we found a significant correlation between changes in geochemical composition and changes in community structure. Differences in organic carbon and mineralogy shaped the relative abundance of microbial taxa. We used correlations to build hypotheses about energy metabolisms, particularly of the Deep Sea Archaeal Group, specific Deltaproteobacteria, and sediment lineages of potentially anaerobic Marine Group I Archaea. We demonstrate that total prokaryotic community structure can be directly correlated to geochemistry within these sediments, thus enhancing our understanding of biogeochemical cycling and our ability to predict metabolisms of uncultured microbes in deep-sea sediments.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Comment in
-
Tracking microbial habitats in subseafloor sediments.Proc Natl Acad Sci U S A. 2012 Oct 16;109(42):16756-7. doi: 10.1073/pnas.1215867109. Epub 2012 Oct 9. Proc Natl Acad Sci U S A. 2012. PMID: 23047701 Free PMC article. No abstract available.
References
-
- Parkes RJ, Cragg BA, Wellsbury P. Recent studies on bacterial populations and processes in subseafloor sediments: A review. Hydrogeol J. 2000;8(1):11–28.
-
- Berner RA. Burial of organic carbon and pyrite sulfur in the modern ocean; its geochemical and environmental significance. Am J Sci. 1982;282(4):451–473.
-
- Rochelle PA, Fry JC, Parkes RJ, Weightman AJ. DNA extraction for 16S ribosomal-RNA gene analysis to determine genetic diversity in deep sediment communities. FEMS Microbiol Lett. 1992;100(1–3):59–65. - PubMed
-
- Marchesi JR, Weightman AJ, Cragg BA, Parkes RJ, Fry JC. Methanogen and bacterial diversity and distribution in deep gas hydrate sediments from the Cascadia Margin as revealed by 16S rRNA molecular analysis. FEMS Microbiol Ecol. 2001;34(3):221–228. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

