Response to hyperosmotic stress
- PMID: 23028184
- PMCID: PMC3454867
- DOI: 10.1534/genetics.112.140863
Response to hyperosmotic stress
Abstract
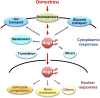
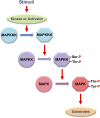
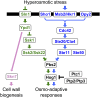
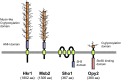
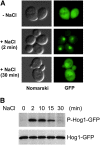
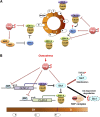
An appropriate response and adaptation to hyperosmolarity, i.e., an external osmolarity that is higher than the physiological range, can be a matter of life or death for all cells. It is especially important for free-living organisms such as the yeast Saccharomyces cerevisiae. When exposed to hyperosmotic stress, the yeast initiates a complex adaptive program that includes temporary arrest of cell-cycle progression, adjustment of transcription and translation patterns, and the synthesis and retention of the compatible osmolyte glycerol. These adaptive responses are mostly governed by the high osmolarity glycerol (HOG) pathway, which is composed of membrane-associated osmosensors, an intracellular signaling pathway whose core is the Hog1 MAP kinase (MAPK) cascade, and cytoplasmic and nuclear effector functions. The entire pathway is conserved in diverse fungal species, while the Hog1 MAPK cascade is conserved even in higher eukaryotes including humans. This conservation is illustrated by the fact that the mammalian stress-responsive p38 MAPK can rescue the osmosensitivity of hog1Δ mutations in response to hyperosmotic challenge. As the HOG pathway is one of the best-understood eukaryotic signal transduction pathways, it is useful not only as a model for analysis of osmostress responses, but also as a model for mathematical analysis of signal transduction pathways. In this review, we have summarized the current understanding of both the upstream signaling mechanism and the downstream adaptive responses to hyperosmotic stress in yeast.
Figures




References
-
- Adrover M., Zi Z., Duch A., Schaber J., González-Novo A., et al. , 2011. Time-dependent quantitative multicomponent control of the G1-S network by the stress-activated protein kinase Hog1 upon osmostress. Sci. Signal. 4: ra63. - PubMed
-
- Aguilera A., 2005. mRNA processing and genomic instability. Nat. Struct. Mol. Biol. 12: 737–738 - PubMed
-
- Aguilera A., Gómez-González B., 2008. Genome instability: a mechanistic view of its causes and consequences. Nat. Rev. Genet. 9: 204–217 - PubMed
-
- Albertyn J., Hohmann S., Thevelein J. M., Prior B. A., 1994. GPD1, which encodes glycerol-3-phosphate dehydrogenase, is essential for growth under osmotic stress in Saccharomyces cerevisiae, and its expression is regulated by the high-osmolarity glycerol response pathway. Mol. Cell. Biol. 14: 4135–4144 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

