The ubiquitin-proteasome system of Saccharomyces cerevisiae
- PMID: 23028185
- PMCID: PMC3454868
- DOI: 10.1534/genetics.112.140467
The ubiquitin-proteasome system of Saccharomyces cerevisiae
Abstract
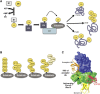
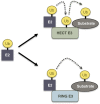
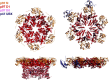
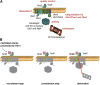
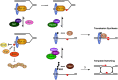
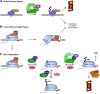
Protein modifications provide cells with exquisite temporal and spatial control of protein function. Ubiquitin is among the most important modifiers, serving both to target hundreds of proteins for rapid degradation by the proteasome, and as a dynamic signaling agent that regulates the function of covalently bound proteins. The diverse effects of ubiquitylation reflect the assembly of structurally distinct ubiquitin chains on target proteins. The resulting ubiquitin code is interpreted by an extensive family of ubiquitin receptors. Here we review the components of this regulatory network and its effects throughout the cell.
Figures



References
-
- Amerik A. Y., Li S. J., Hochstrasser M., 2000b Analysis of the deubiquitinating enzymes of the yeast Saccharomyces cerevisiae. Biol. Chem. 381: 981–992 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

