Influence of charge on FITC-BSA-loaded chondroitin sulfate-chitosan nanoparticles upon cell uptake in human Caco-2 cell monolayers
- PMID: 23028215
- PMCID: PMC3441231
- DOI: 10.2147/IJN.S34770
Influence of charge on FITC-BSA-loaded chondroitin sulfate-chitosan nanoparticles upon cell uptake in human Caco-2 cell monolayers
Abstract
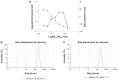
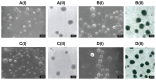
Background and methods: Chondroitin sulfate-chitosan (ChS-CS) nanoparticles and positively and negatively charged fluorescein isothiocyanate-conjugated bovine serum albumin (FITC-BSA)-loaded ChS-CS nanoparticles were prepared and characterized. The properties of ChS-CS nanoparticles, including cellular uptake, cytotoxicity, and transepithelial transport, as well as findings on field emission-scanning electron microscopy, transmission electron microscopy, and confocal laser scanning microscopy were evaluated in human epithelial colorectal adenocarcinoma (Caco-2) fibroblasts. ChS-CS nanoparticles with a mean particle size of 250 nm and zeta potentials ranging from -30 to +18 mV were prepared using an ionic gelation method.
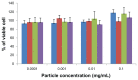
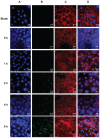
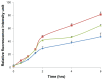
Results: Standard cell viability assays demonstrated that cells incubated with ChS-CS and FITC-BSA-loaded ChS-CS nanoparticles remained more than 95% viable at particle concentrations up to 0.1 mg/mL. Endocytosis of nanoparticles was confirmed by confocal laser scanning microscopy and measured by flow cytometry. Ex vivo transepithelial transport studies using Caco-2 cells indicated that the nanoparticles were effectively transported into Caco-2 cells via endocytosis. The uptake of positively charged FITC-BSA-loaded ChS-CS nanoparticles across the epithelial membrane was more efficient than that of the negatively charged nanoparticles.
Conclusion: The ChS-CS nanoparticles fabricated in this study were effectively endocytosed by Caco-2 fibroblasts without significant cytotoxicity at high nanoparticle concentrations. ChS-CS nanoparticles represent a potential novel delivery system for the transport of hydrophilic macromolecules.
Keywords: cell uptake; chitosan; chondroitin sulfate; cytotoxicity; nanoparticles.
Figures
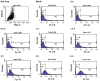
 ), negatively charged FITC-BSA ChS-CS nanoparticles (
), negatively charged FITC-BSA ChS-CS nanoparticles (
 ), and FITC-BSA solution (0.1 mL; 0.1 mg/mL) (
), and FITC-BSA solution (0.1 mL; 0.1 mg/mL) (
 ) on Caco-2 cells at varied time incubations. Note: Results are represented as the mean ± standard deviation, n = 6. Abbreviations: BSA, bovine serum albumin; ChS, chondroitin 4-sulfate sodium salt; CS, chitosan; FITC, fluorescein isothiocyanate.
) on Caco-2 cells at varied time incubations. Note: Results are represented as the mean ± standard deviation, n = 6. Abbreviations: BSA, bovine serum albumin; ChS, chondroitin 4-sulfate sodium salt; CS, chitosan; FITC, fluorescein isothiocyanate.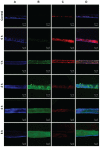
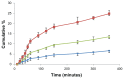
 ); negative charge of FITC-BSA-loaded ChS-CS nanoparticles (
); negative charge of FITC-BSA-loaded ChS-CS nanoparticles (
 ), and FITC-BSA solution (0.1 mL; 0.1 mg/mL) (
), and FITC-BSA solution (0.1 mL; 0.1 mg/mL) (
 ). Notes: All values are shown as the mean ± standard deviation; n = 6. Abbreviations: BSA, bovine serum albumin; ChS, chondroitin 4-sulfate sodium salt; CS, chitosan; FITC, fluorescein isothiocyanate.
). Notes: All values are shown as the mean ± standard deviation; n = 6. Abbreviations: BSA, bovine serum albumin; ChS, chondroitin 4-sulfate sodium salt; CS, chitosan; FITC, fluorescein isothiocyanate.Similar articles
-
Novel protein-loaded chondroitin sulfate-chitosan nanoparticles: preparation and characterization.Acta Biomater. 2011 Oct;7(10):3804-12. doi: 10.1016/j.actbio.2011.06.026. Epub 2011 Jun 24. Acta Biomater. 2011. PMID: 21742066
-
Positively and negatively surface-charged chondroitin sulfate-trimethylchitosan nanoparticles as protein carriers.Carbohydr Polym. 2016 Feb 10;137:532-540. doi: 10.1016/j.carbpol.2015.10.095. Epub 2015 Nov 4. Carbohydr Polym. 2016. PMID: 26686160
-
Methylated N-(4-N,N-dimethylaminobenzyl) chitosan coated liposomes for oral protein drug delivery.Eur J Pharm Sci. 2012 Sep 29;47(2):359-66. doi: 10.1016/j.ejps.2012.06.020. Epub 2012 Jul 6. Eur J Pharm Sci. 2012. PMID: 22771544
-
Biomedical and Pharmacological Uses of Fluorescein Isothiocyanate Chitosan-Based Nanocarriers.Macromol Biosci. 2021 Jan;21(1):e2000312. doi: 10.1002/mabi.202000312. Epub 2020 Oct 4. Macromol Biosci. 2021. PMID: 33016007 Review.
-
Chondroitin sulfate, hyaluronic acid and chitin/chitosan production using marine waste sources: characteristics, applications and eco-friendly processes: a review.Mar Drugs. 2013 Mar 11;11(3):747-74. doi: 10.3390/md11030747. Mar Drugs. 2013. PMID: 23478485 Free PMC article. Review.
Cited by
-
Novel Anthra[1,2-c][1,2,5]Thiadiazole-6,11-Diones as Promising Anticancer Lead Compounds: Biological Evaluation, Characterization & Molecular Targets Determination.PLoS One. 2016 Apr 21;11(4):e0154278. doi: 10.1371/journal.pone.0154278. eCollection 2016. PLoS One. 2016. PMID: 27100886 Free PMC article.
-
Biocompatibility and Stability of Polysaccharide Polyelectrolyte Complexes Aimed at Respiratory Delivery.Materials (Basel). 2015 Aug 28;8(9):5647-5670. doi: 10.3390/ma8095268. Materials (Basel). 2015. PMID: 28793528 Free PMC article.
-
Real time observation of the interaction between aluminium salts and sweat under microfluidic conditions.Sci Rep. 2021 Mar 18;11(1):6376. doi: 10.1038/s41598-021-85691-8. Sci Rep. 2021. PMID: 33737654 Free PMC article.
-
Liposome-polyethylenimine complexes for the effective delivery of HuR siRNA in the treatment of diabetic retinopathy.Drug Deliv Transl Res. 2023 Jun;13(6):1675-1698. doi: 10.1007/s13346-022-01281-9. Epub 2023 Jan 11. Drug Deliv Transl Res. 2023. PMID: 36630075
-
Novel targeting using nanoparticles: an approach to the development of an effective anti-leishmanial drug-delivery system.Int J Nanomedicine. 2014 Feb 14;9:877-90. doi: 10.2147/IJN.S55678. eCollection 2014. Int J Nanomedicine. 2014. PMID: 24627630 Free PMC article.
References
-
- Chadwick S, Kriegel C, Amiji M. Nanotechnology solutions for mucosal immunization. Adv Drug Deliv Rev. 2010;62(4–5):394–407. - PubMed
-
- Camargo JA, Sapin A, Daloz D, Maincent P. Ivermectin-loaded microparticles for parenteral sustained release: in vitro characterization and effect of some formulation variables. J Microencapsul. 2010;27(7):609–617. - PubMed
-
- Li Z, Zhang L, Sun W, Ding Q, Hou Y, Xu Y. Archaeosomes with encapsulated antigens for oral vaccine delivery. Vaccine. 2011;29(32):5260–5266. - PubMed
-
- Win KY, Feng SS. Effects of particle size and surface coating on cellular uptake of polymeric nanoparticles for oral delivery of anticancer drugs. Biomaterials. 2005;26(15):2713–2722. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

