CD44+/CD105+ human amniotic fluid mesenchymal stem cells survive and proliferate in the ovary long-term in a mouse model of chemotherapy-induced premature ovarian failure
- PMID: 23028242
- PMCID: PMC3461764
- DOI: 10.7150/ijms.4841
CD44+/CD105+ human amniotic fluid mesenchymal stem cells survive and proliferate in the ovary long-term in a mouse model of chemotherapy-induced premature ovarian failure
Abstract
Objectives: Stem cell transplantation has been reported to rescue ovarian function in a preclinical mouse model of chemotherapy-induced premature ovarian failure (POF); however, maintaining the survival and self-renewal of transplanted seed cells in ovarian tissues over the long-term remains a troublesome issue. In this study we aimed to determine whether the CD44+/CD105+ human amniotic fluid cell (HuAFCs) subpopulation represent potential seed cells for stem cell transplantation treatments in POF.
Materials and methods: The CD44+/CD105+ subpopulation were isolated from HuAFCs, cultured in vitro, and injected into a cyclophosphamide-induced mouse model of POF.
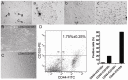
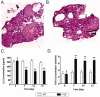
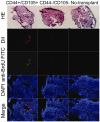
Results: Under continuous subculture in vitro, CD44+/CD105+ cells proliferated rapidly and expressed high levels of the proliferative markers Ki67 and survivin, as well as high levels of a number of mesenchymal stem cell biomarkers. Moreover, when red fluorescence protein (RFP)-transduced CD44+/CD105+ HuAFCs were transplanted into the ovaries of POF mice, the cells could be detected by fluorescence microscopy up to three weeks after injection. Furthermore, the BrdUrd incorporation assay and immunofluorescent staining demonstrated that CD44+/CD105+ HuAFCs underwent normal cycles of cell proliferation and self-renewal in the ovarian tissues of POF mice over the long-term.
Conclusions: The mesenchymal stem cell properties and long-term in vivo survival of CD44+/CD105+ HuAFCs make them ideal seed cells for stem cell transplantation to treat POF.
Keywords: Mouse premature ovarian failure model; cell transplantation; human amniotic fluid cells; long-term survival; mesenchymal stem cell like cells; proliferation..
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures


References
-
- Bandyopadhyay S, Chakrabarti J, Banerjee S, Pal AK, Goswami SK, Chakravarty BN. et al. Galactose toxicity in the rat as a model for premature ovarian failure: an experimental approach readdressed. Hum Reprod. 2003;18:2031–2038. - PubMed
-
- Duncan M, Cummings L, Chada K. Germ cell deficient (gcd) mouse as a model of premature ovarian failure. Biol Reprod. 1993;49:221–227. - PubMed
-
- Lee HJ, Selesniemi K, Niikura Y, Niikura T, Klein R, Dombkowski DM. et al. Bone marrow transplantation generates immature oocytes and rescues long-term fertility in a preclinical mouse model of chemotherapy-induced premature ovarian failure. J Clin Oncol. 2007;25:3198–3204. - PubMed
-
- Yucebilgin MS, Terek MC, Ozsaran A, Akercan F, Zekioglu O, Isik E. et al. Effect of chemotherapy on primordial follicular reserve of rat: an animal model of premature ovarian failure and infertility. Aust N Z J Obstet Gynaecol. 2004;44:6–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

