The Legionella IcmSW complex directly interacts with DotL to mediate translocation of adaptor-dependent substrates
- PMID: 23028312
- PMCID: PMC3441705
- DOI: 10.1371/journal.ppat.1002910
The Legionella IcmSW complex directly interacts with DotL to mediate translocation of adaptor-dependent substrates
Abstract
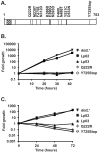
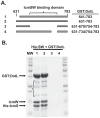
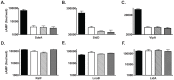
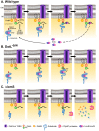
Legionella pneumophila is a Gram-negative bacterium that replicates within human alveolar macrophages by evasion of the host endocytic pathway through the formation of a replicative vacuole. Generation of this vacuole is dependent upon the secretion of over 275 effector proteins into the host cell via the Dot/Icm type IVB secretion system (T4SS). The type IV coupling protein (T4CP) subcomplex, consisting of DotL, DotM, DotN, IcmS and IcmW, was recently defined. DotL is proposed to be the T4CP of the L. pneumophila T4SS based on its homology to known T4CPs, which function as inner-membrane receptors for substrates. As a result, DotL is hypothesized to play an integral role(s) in the L. pneumophila T4SS for the engagement and translocation of substrates. To elucidate this role, a genetic approach was taken to screen for dotL mutants that were unable to survive inside host cells. One mutant, dotLY725Stop, did not interact with the type IV adaptor proteins IcmS/IcmW (IcmSW) leading to the identification of an IcmSW-binding domain on DotL. Interestingly, the dotLY725Stop mutant was competent for export of one class of secreted effectors, the IcmSW-independent substrates, but exhibited a specific defect in secretion of IcmSW-dependent substrates. This differential secretion illustrates that DotL requires a direct interaction with the type IV adaptor proteins for the secretion of a major class of substrates. Thus, by identifying a new target for IcmSW, we have discovered that the type IV adaptors perform an additional role in the export of substrates by the L. pneumophila Dot/Icm T4SS.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




Similar articles
-
Identification of the DotL coupling protein subcomplex of the Legionella Dot/Icm type IV secretion system.Mol Microbiol. 2012 Jul;85(2):378-91. doi: 10.1111/j.1365-2958.2012.08118.x. Epub 2012 Jun 14. Mol Microbiol. 2012. PMID: 22694730 Free PMC article.
-
The Legionella pneumophila IcmSW complex interacts with multiple Dot/Icm effectors to facilitate type IV translocation.PLoS Pathog. 2007 Dec;3(12):e188. doi: 10.1371/journal.ppat.0030188. PLoS Pathog. 2007. PMID: 18069892 Free PMC article.
-
Structural insights into the roles of the IcmS-IcmW complex in the type IVb secretion system of Legionella pneumophila.Proc Natl Acad Sci U S A. 2017 Dec 19;114(51):13543-13548. doi: 10.1073/pnas.1706883115. Epub 2017 Dec 4. Proc Natl Acad Sci U S A. 2017. PMID: 29203674 Free PMC article.
-
Effector translocation by the Legionella Dot/Icm type IV secretion system.Curr Top Microbiol Immunol. 2013;376:103-15. doi: 10.1007/82_2013_345. Curr Top Microbiol Immunol. 2013. PMID: 23918176 Review.
-
Identification of legionella effectors using bioinformatic approaches.Methods Mol Biol. 2013;954:595-602. doi: 10.1007/978-1-62703-161-5_37. Methods Mol Biol. 2013. PMID: 23150423 Review.
Cited by
-
Structural organisation of the type IV secretion systems.Curr Opin Microbiol. 2014 Feb;17(100):24-31. doi: 10.1016/j.mib.2013.11.001. Epub 2013 Dec 5. Curr Opin Microbiol. 2014. PMID: 24581689 Free PMC article. Review.
-
Chimeric Coupling Proteins Mediate Transfer of Heterologous Type IV Effectors through the Escherichia coli pKM101-Encoded Conjugation Machine.J Bacteriol. 2016 Sep 9;198(19):2701-18. doi: 10.1128/JB.00378-16. Print 2016 Oct 1. J Bacteriol. 2016. PMID: 27432829 Free PMC article.
-
A Versatile Nanoluciferase Reporter Reveals Structural Properties Associated with a Highly Efficient, N-Terminal Legionella pneumophila Type IV Secretion Translocation Signal.Microbiol Spectr. 2023 Feb 23;11(2):e0233822. doi: 10.1128/spectrum.02338-22. Online ahead of print. Microbiol Spectr. 2023. PMID: 36815834 Free PMC article.
-
Architecture of the type IV coupling protein complex of Legionella pneumophila.Nat Microbiol. 2017 Jul 17;2:17114. doi: 10.1038/nmicrobiol.2017.114. Nat Microbiol. 2017. PMID: 28714967 Free PMC article.
-
Pathogenicity and Virulence of Legionella: Intracellular replication and host response.Virulence. 2021 Dec;12(1):1122-1144. doi: 10.1080/21505594.2021.1903199. Virulence. 2021. PMID: 33843434 Free PMC article. Review.
References
-
- McDade JE, Shepard CC, Fraser DW, Tsai TR, Redus MA, et al. (1977) Legionnaires' disease: isolation of a bacterium and demonstration of its role in other respiratory disease. N Engl J Med 297: 1197–1203. - PubMed
-
- Hubber A, Roy CR (2010) Modulation of host cell function by Legionella pneumophila type IV effectors. Annu Rev Cell Dev Biol 26: 261–283. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

