Identification of alternatively translated Tetherin isoforms with differing antiviral and signaling activities
- PMID: 23028328
- PMCID: PMC3460627
- DOI: 10.1371/journal.ppat.1002931
Identification of alternatively translated Tetherin isoforms with differing antiviral and signaling activities
Abstract
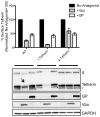
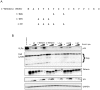
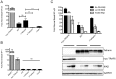
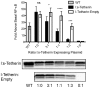
Tetherin (BST-2/CD317/HM1.24) is an IFN induced transmembrane protein that restricts release of a broad range of enveloped viruses. Important features required for Tetherin activity and regulation reside within the cytoplasmic domain. Here we demonstrate that two isoforms, derived by alternative translation initiation from highly conserved methionine residues in the cytoplasmic domain, are produced in both cultured human cell lines and primary cells. These two isoforms have distinct biological properties. The short isoform (s-Tetherin), which lacks 12 residues present in the long isoform (l-Tetherin), is significantly more resistant to HIV-1 Vpu-mediated downregulation and consequently more effectively restricts HIV-1 viral budding in the presence of Vpu. s-Tetherin Vpu resistance can be accounted for by the loss of serine-threonine and tyrosine motifs present in the long isoform. By contrast, the l-Tetherin isoform was found to be an activator of nuclear factor-kappa B (NF-κB) signaling whereas s-Tetherin does not activate NF-κB. Activation of NF-κB requires a tyrosine-based motif found within the cytoplasmic tail of the longer species and may entail formation of l-Tetherin homodimers since co-expression of s-Tetherin impairs the ability of the longer isoform to activate NF-κB. These results demonstrate a novel mechanism for control of Tetherin antiviral and signaling function and provide insight into Tetherin function both in the presence and absence of infection.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

