A unique protein phosphatase with kelch-like domains (PPKL) in Plasmodium modulates ookinete differentiation, motility and invasion
- PMID: 23028336
- PMCID: PMC3447748
- DOI: 10.1371/journal.ppat.1002948
A unique protein phosphatase with kelch-like domains (PPKL) in Plasmodium modulates ookinete differentiation, motility and invasion
Abstract
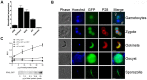
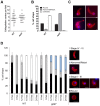
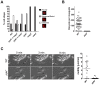
Protein phosphorylation and dephosphorylation (catalysed by kinases and phosphatases, respectively) are post-translational modifications that play key roles in many eukaryotic signalling pathways, and are often deregulated in a number of pathological conditions in humans. In the malaria parasite Plasmodium, functional insights into its kinome have only recently been achieved, with over half being essential for blood stage development and another 14 kinases being essential for sexual development and mosquito transmission. However, functions for any of the plasmodial protein phosphatases are unknown. Here, we use reverse genetics in the rodent malaria model, Plasmodium berghei, to examine the role of a unique protein phosphatase containing kelch-like domains (termed PPKL) from a family related to Arabidopsis BSU1. Phylogenetic analysis confirmed that the family of BSU1-like proteins including PPKL is encoded in the genomes of land plants, green algae and alveolates, but not in other eukaryotic lineages. Furthermore, PPKL was observed in a distinct family, separate to the most closely-related phosphatase family, PP1. In our genetic approach, C-terminal GFP fusion with PPKL showed an active protein phosphatase preferentially expressed in female gametocytes and ookinetes. Deletion of the endogenous ppkl gene caused abnormal ookinete development and differentiation, and dissociated apical microtubules from the inner-membrane complex, generating an immotile phenotype and failure to invade the mosquito mid-gut epithelium. These observations were substantiated by changes in localisation of cytoskeletal tubulin and actin, and the micronemal protein CTRP in the knockout mutant as assessed by indirect immunofluorescence. Finally, increased mRNA expression of dozi, a RNA helicase vital to zygote development was observed in ppkl(-) mutants, with global phosphorylation studies of ookinete differentiation from 1.5-24 h post-fertilisation indicating major changes in the first hours of zygote development. Our work demonstrates a stage-specific essentiality of the unique PPKL enzyme, which modulates parasite differentiation, motility and transmission.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Graves JD, Krebs EG (1999) Protein phosphorylation and signal transduction. Pharmacol Ther 82: 111–121. - PubMed
-
- Blume-Jensen P, Hunter T (2001) Oncogenic kinase signalling. Nature 411: 355–365. - PubMed
-
- Tan CS, Bodenmiller B, Pasculescu A, Jovanovic M, Hengartner MO, et al. (2009) Comparative analysis reveals conserved protein phosphorylation networks implicated in multiple diseases. Sci Signal 2: ra39. - PubMed
-
- Barford D, Das AK, Egloff MP (1998) The structure and mechanism of protein phosphatases: insights into catalysis and regulation. Annu Rev Biophys Biomol Struct 27: 133–164. - PubMed
-
- Gallego M, Virshup DM (2005) Protein serine/threonine phosphatases: life, death, and sleeping. Curr Opin Cell Biol 17: 197–202. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

