Tetraspanin is required for generation of reactive oxygen species by the dual oxidase system in Caenorhabditis elegans
- PMID: 23028364
- PMCID: PMC3447965
- DOI: 10.1371/journal.pgen.1002957
Tetraspanin is required for generation of reactive oxygen species by the dual oxidase system in Caenorhabditis elegans
Abstract
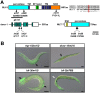
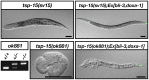
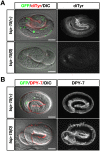
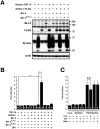
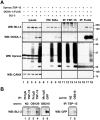
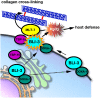
Reactive oxygen species (ROS) are toxic but essential molecules responsible for host defense and cellular signaling. Conserved NADPH oxidase (NOX) family enzymes direct the regulated production of ROS. Hydrogen peroxide (H(2)O(2)) generated by dual oxidases (DUOXs), a member of the NOX family, is crucial for innate mucosal immunity. In addition, H(2)O(2) is required for cellular signaling mediated by protein modifications, such as the thyroid hormone biosynthetic pathway in mammals. In contrast to other NOX isozymes, the regulatory mechanisms of DUOX activity are less understood. Using Caenorhabditis elegans as a model, we demonstrate that the tetraspanin protein is required for induction of the DUOX signaling pathway in conjunction with the dual oxidase maturation factor (DUOXA). In the current study, we show that genetic mutation of DUOX (bli-3), DUOXA (doxa-1), and peroxidase (mlt-7) in C. elegans causes the same defects as a tetraspanin tsp-15 mutant, represented by exoskeletal deficiencies due to the failure of tyrosine cross-linking of collagen. The deficiency in the tsp-15 mutant was restored by co-expression of bli-3 and doxa-1, indicating the involvement of tsp-15 in the generation of ROS. H(2)O(2) generation by BLI-3 was completely dependent on TSP-15 when reconstituted in mammalian cells. We also demonstrated that TSP-15, BLI-3, and DOXA-1 form complexes in vitro and in vivo. Cell-fusion-based analysis suggested that association with TSP-15 at the cell surface is crucial for BLI-3 activation to release H(2)O(2). This study provides the first evidence for an essential role of tetraspanin in ROS generation.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Bedard K, Krause K-H (2007) The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 87: 245–313. - PubMed
-
- Leto TL, Geiszt M (2006) Role of Nox family NADPH oxidases in host defense. Antioxid Redox Signal 8: 1549–1561. - PubMed
-
- Lambeth JD (2004) NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol 4: 181–189. - PubMed
-
- Sumimoto H (2008) Structure, regulation and evolution of Nox-family NADPH oxidases that produce reactive oxygen species. FEBS J 275: 3249–3277. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

