De novo CNV formation in mouse embryonic stem cells occurs in the absence of Xrcc4-dependent nonhomologous end joining
- PMID: 23028374
- PMCID: PMC3447954
- DOI: 10.1371/journal.pgen.1002981
De novo CNV formation in mouse embryonic stem cells occurs in the absence of Xrcc4-dependent nonhomologous end joining
Abstract
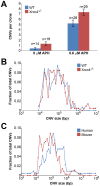
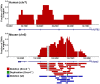
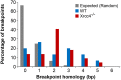
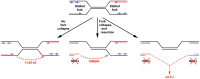
Spontaneous copy number variant (CNV) mutations are an important factor in genomic structural variation, genomic disorders, and cancer. A major class of CNVs, termed nonrecurrent CNVs, is thought to arise by nonhomologous DNA repair mechanisms due to the presence of short microhomologies, blunt ends, or short insertions at junctions of normal and de novo pathogenic CNVs, features recapitulated in experimental systems in which CNVs are induced by exogenous replication stress. To test whether the canonical nonhomologous end joining (NHEJ) pathway of double-strand break (DSB) repair is involved in the formation of this class of CNVs, chromosome integrity was monitored in NHEJ-deficient Xrcc4(-/-) mouse embryonic stem (ES) cells following treatment with low doses of aphidicolin, a DNA replicative polymerase inhibitor. Mouse ES cells exhibited replication stress-induced CNV formation in the same manner as human fibroblasts, including the existence of syntenic hotspot regions, such as in the Auts2 and Wwox loci. The frequency and location of spontaneous and aphidicolin-induced CNV formation were not altered by loss of Xrcc4, as would be expected if canonical NHEJ were the predominant pathway of CNV formation. Moreover, de novo CNV junctions displayed a typical pattern of microhomology and blunt end use that did not change in the absence of Xrcc4. A number of complex CNVs were detected in both wild-type and Xrcc4(-/-) cells, including an example of a catastrophic, chromothripsis event. These results establish that nonrecurrent CNVs can be, and frequently are, formed by mechanisms other than Xrcc4-dependent NHEJ.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Iafrate AJ, Feuk L, Rivera MN, Listewnik ML, Donahoe PK, et al. (2004) Detection of large-scale variation in the human genome. Nature Genetics 36: 949–951. - PubMed
-
- Sebat J, Lakshmi B, Troge J, Alexander J, Young J, et al. (2004) Large-scale copy number polymorphism in the human genome. Science 305: 525–528. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

