Manipulating fatty acid biosynthesis in microalgae for biofuel through protein-protein interactions
- PMID: 23028438
- PMCID: PMC3441505
- DOI: 10.1371/journal.pone.0042949
Manipulating fatty acid biosynthesis in microalgae for biofuel through protein-protein interactions
Abstract
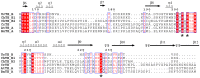
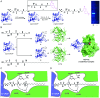
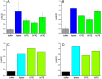
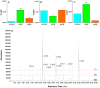
Microalgae are a promising feedstock for renewable fuels, and algal metabolic engineering can lead to crop improvement, thus accelerating the development of commercially viable biodiesel production from algae biomass. We demonstrate that protein-protein interactions between the fatty acid acyl carrier protein (ACP) and thioesterase (TE) govern fatty acid hydrolysis within the algal chloroplast. Using green microalga Chlamydomonas reinhardtii (Cr) as a model, a structural simulation of docking CrACP to CrTE identifies a protein-protein recognition surface between the two domains. A virtual screen reveals plant TEs with similar in silico binding to CrACP. Employing an activity-based crosslinking probe designed to selectively trap transient protein-protein interactions between the TE and ACP, we demonstrate in vitro that CrTE must functionally interact with CrACP to release fatty acids, while TEs of vascular plants show no mechanistic crosslinking to CrACP. This is recapitulated in vivo, where overproduction of the endogenous CrTE increased levels of short-chain fatty acids and engineering plant TEs into the C. reinhardtii chloroplast did not alter the fatty acid profile. These findings highlight the critical role of protein-protein interactions in manipulating fatty acid biosynthesis for algae biofuel engineering as illuminated by activity-based probes.
Conflict of interest statement
Figures


References
-
- Chisti Y (2007) Biodiesel from microalgae. Biotechnology Advances 25: 294–306. - PubMed
-
- Scott SA, Davey MP, Dennis JS, Horst I, Howe CJ, et al. (2010) Biodiesel from algae: challenges and prospects. Current Opinion in Biotechnology 21: 277–286. - PubMed
-
- Hu Q, Sommerfeld M, Jarvis E, Ghirardi M, Posewitz M, et al. (2008) Microalgal triacylglycerols as feedstocks for biofuel production: perspectives and advances. Plant Journal 54: 621–639. - PubMed
-
- Durrett TP, Benning C, Ohlrogge J (2008) Plant triacylglycerols as feedstocks for the production of biofuels. Plant Journal 54: 593–607. - PubMed
-
- Mata TM, Martins AA, Caetano NS (2010) Microalgae for biodiesel production and other applications: A review. Renewable and Sustainable Energy Reviews 14: 217–232.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

