Drosophila Ninjurin A induces nonapoptotic cell death
- PMID: 23028562
- PMCID: PMC3460944
- DOI: 10.1371/journal.pone.0044567
Drosophila Ninjurin A induces nonapoptotic cell death
Abstract
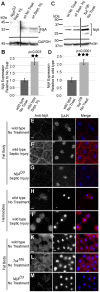
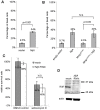
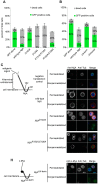
Ninjurins are conserved transmembrane proteins that are upregulated across species in response to injury and stress. Their biological functions are not understood, in part because there have been few in vivo studies of their function. We analyzed the expression and function of one of three Drosophila Ninjurins, NijA. We found that NijA protein is redistributed to the cell surface in larval immune tissues after septic injury and is upregulated by the Toll pathway. We generated a null mutant of NijA, which displayed no detectable phenotype. In ectopic expression studies, NijA induced cell death, as evidenced by cell loss and acridine orange staining. These dying cells did not display hallmarks of apoptotic cells including TUNEL staining and inhibition by p35, indicating that NijA induced nonapoptotic cell death. In cell culture, NijA also induced cell death, which appeared to be cell autonomous. These in vivo studies identify a new role for the Ninjurin family in inducing nonapoptotic cell death.
Conflict of interest statement
Figures



Similar articles
-
An MMP liberates the Ninjurin A ectodomain to signal a loss of cell adhesion.Genes Dev. 2006 Jul 15;20(14):1899-910. doi: 10.1101/gad.1426906. Epub 2006 Jun 30. Genes Dev. 2006. PMID: 16815999 Free PMC article.
-
Draper-mediated and phosphatidylserine-independent phagocytosis of apoptotic cells by Drosophila hemocytes/macrophages.J Biol Chem. 2004 Nov 12;279(46):48466-76. doi: 10.1074/jbc.M408597200. Epub 2004 Sep 1. J Biol Chem. 2004. PMID: 15342648
-
JNK pathway activation is able to synchronize neuronal death and glial phagocytosis in Drosophila.Cell Death Dis. 2015 Feb 19;6(2):e1649. doi: 10.1038/cddis.2015.27. Cell Death Dis. 2015. PMID: 25695602 Free PMC article.
-
The genetics of hiding the corpse: engulfment and degradation of apoptotic cells in C. elegans and D. melanogaster.Curr Top Dev Biol. 2004;63:91-143. doi: 10.1016/S0070-2153(04)63004-3. Curr Top Dev Biol. 2004. PMID: 15536015 Review. No abstract available.
-
Nonapoptotic Cell Death Pathways.Cold Spring Harb Perspect Biol. 2022 Nov 1;14(11):a041079. doi: 10.1101/cshperspect.a041079. Cold Spring Harb Perspect Biol. 2022. PMID: 36319068 Free PMC article. Review. No abstract available.
Cited by
-
Gasdermins in Innate Host Defense Against Entamoeba histolytica and Other Protozoan Parasites.Front Immunol. 2022 Jun 20;13:900553. doi: 10.3389/fimmu.2022.900553. eCollection 2022. Front Immunol. 2022. PMID: 35795683 Free PMC article. Review.
-
Unity in defence: honeybee workers exhibit conserved molecular responses to diverse pathogens.BMC Genomics. 2017 Mar 2;18(1):207. doi: 10.1186/s12864-017-3597-6. BMC Genomics. 2017. PMID: 28249569 Free PMC article.
-
Tsetse fly tolerance to T. brucei infection: transcriptome analysis of trypanosome-associated changes in the tsetse fly salivary gland.BMC Genomics. 2016 Nov 25;17(1):971. doi: 10.1186/s12864-016-3283-0. BMC Genomics. 2016. PMID: 27884110 Free PMC article.
-
The transcriptional response in mosquitoes distinguishes between fungi and bacteria but not Gram types.BMC Genomics. 2024 Apr 9;25(1):353. doi: 10.1186/s12864-024-10153-0. BMC Genomics. 2024. PMID: 38594632 Free PMC article.
-
Non-Target Effects of Green Fluorescent Protein (GFP)-Derived Double-Stranded RNA (dsRNA-GFP) Used in Honey Bee RNA Interference (RNAi) Assays.Insects. 2013 Jan 4;4(1):90-103. doi: 10.3390/insects4010090. Insects. 2013. PMID: 26466797 Free PMC article.
References
-
- Araki T, Milbrandt J (1996) Ninjurin, a novel adhesion molecule, is induced by nerve injury and promotes axonal growth. Neuron 17: 353–361. - PubMed
-
- Koike M, Ninomiya Y, Koike A (2008) Characterization of Ninjurin and TSC22 induction after X-irradiation of normal human skin cells. The Journal of dermatology 35: 6–17. - PubMed
-
- Boutros M, Agaisse H, Perrimon N (2002) Sequential activation of signaling pathways during innate immune responses in Drosophila. Dev Cell 3: 711–722. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

