Host platelets and, in part, neutrophils mediate lung accumulation of transfused UVB-irradiated human platelets in a mouse model of acute lung injury
- PMID: 23028636
- PMCID: PMC3446987
- DOI: 10.1371/journal.pone.0044829
Host platelets and, in part, neutrophils mediate lung accumulation of transfused UVB-irradiated human platelets in a mouse model of acute lung injury
Abstract
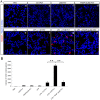
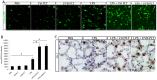
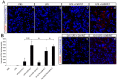
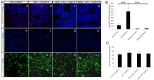
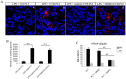
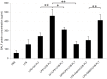
We previously reported that ultraviolet light B (UVB)-treated human platelets (hPLTs) can cause acute lung injury (ALI) in a two-event SCID mouse model in which the predisposing event was Lipopolysaccharide (LPS) injection and the second event was infusion of UVB-treated hPLTs. To delineate contributions of host mouse platelets (mPLTs) and neutrophils in the pathogenesis of ALI in this mouse model, we depleted mPLTs or neutrophils and measured hPLT accumulation in the lung. We also assessed lung injury by protein content in bronchoalveolar lavage fluid (BALF). LPS injection followed by infusion of UVB-treated hPLTs resulted in sequestration of both mPLTs and hPLTs in the lungs of SCID mice, although the numbers of neutrophils in the lung were not significantly different from the control group. Depletion of mouse neutrophils caused only a mild reduction in UVB-hPLTs accumulation in the lungs and a mild reduction in protein content in BALF. In comparison, depletion of mPLTs almost completely abolished hPLTs accumulation in the lung and significantly reduced protein content in BALF. UVB-treated hPLTs bound to host mPLTs, but did not bind to neutrophils in the lung. Aspirin treatment of hPLTs in vitro abolished hPLT accumulation in the lung and protected mice from lung injury. Our data indicate that host mPLTs accumulated in the lungs in response to an inflammatory challenge and subsequently mediated the attachment of transfused UVB-hPLTs. Neutrophils also recruited a small percentage of platelets to the lung. These findings may help develop therapeutic strategies for ALI which could potentially result from transfusion of UV illuminated platelets.
Conflict of interest statement
Figures


References
-
- Heal JM, Blumberg N (2004) Optimizing platelet transfusion therapy. Blood Rev 18: 149–165. - PubMed
-
- Kleinman S, Caulfield T, Chan P, Davenport R, McFarland J, et al. (2004) Toward an understanding of transfusion-related acute lung injury: statement of a consensus panel. Transfusion 44: 1774–1789. - PubMed
-
- Silliman CC, Paterson AJ, Dickey WO, Stroneck DF, Popovsky MA, et al. (1997) The association of biologically active lipids with the development of transfusion-related acute lung injury: a retrospective study. Transfusion 37: 719–726. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

